Angiopoietin / Tie2 Receptor-Ligand System
The Tie receptor tyrosine kinases and their angiopoietin ligands play central roles in developmental and tumor-induced angiogenesis. Although highly homologous, the different angiopoietin family members elicit distinct responses in a context-dependent manner, and the molecular mechanisms of this phenomenon remain unclear. It was originally concluded that angiopoietins signaled exclusively through the Tie2 receptor tyrosine kinase. However, it has more recently been shown that both the highly related Tie1 as well as the heterodimeric a5b1 integrin receptor each play a role in angiopoietin signaling. How these proteins function remains a mystery and is the subject of our investigations. We propose to extend our present understanding of angiopoietin signaling by using the tools of X-ray crystallography combined with other biochemical and biophysical techniques to examine how these molecules associate with each other at the molecular level. Furthermore, using mRNA display, we will optimize the affinity of our structure-based peptide inhibitor of Ang2/Tie2 interactions. Therefore, our results will have both fundamental as well as applied applications. The specific aims of our proposed experiments are:
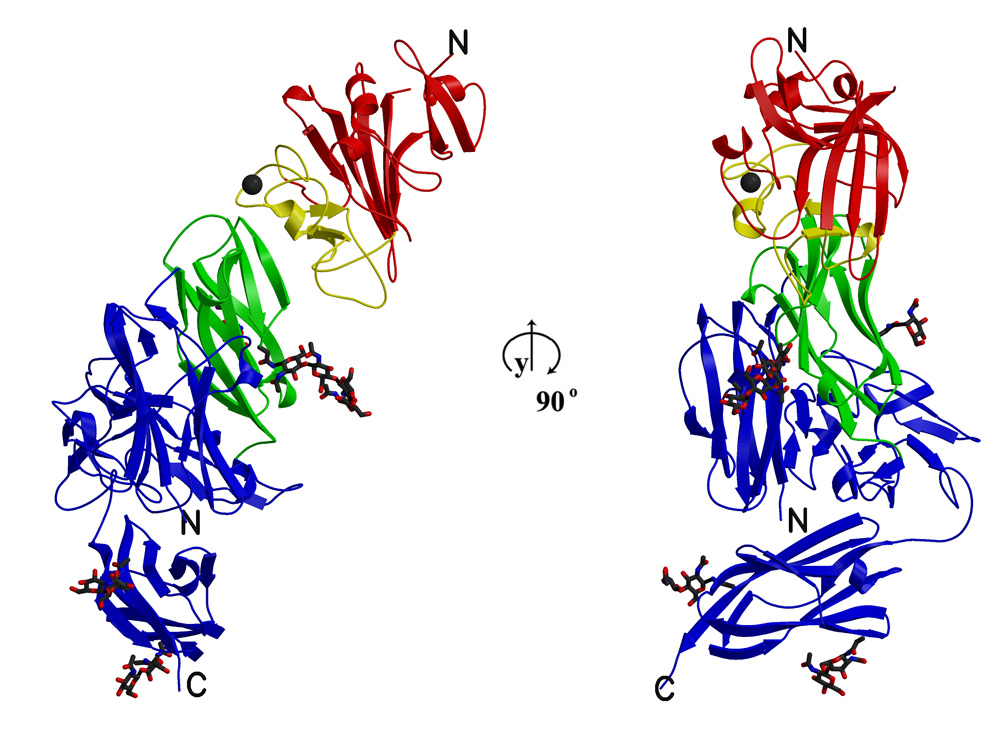
Structural characterization of a signaling competent Angiopoietin/Tie2 complex.
We have previously determined the crystal structure of the Angiopoietin-2 receptor-binding domain containing the carboxy-terminal fibrinogen (receptor-binding) domain but lacking the functionally essential amino terminal coiled-coil and superclustering domains. We have further determined the structure of the complex of this domain bound to the Tie2 ligand-binding domain and shown that ligand recognition proceeds via a lock-and-key mechanism and in this regard, is similar to that observed in antibody-ligand binding. Our structure was, however, limited to a signaling incompetent heterodimeric complex of the two molecules, and our results, as well as others, have shown that receptor clustering is essential for receptor activation. Therefore, we will build upon our present structural understanding of Angiopoietins by determining the three-dimensional structure of a full-length Angiopoietin family member in association with Tie2, thus providing a structural basis for receptor clustering and activation.
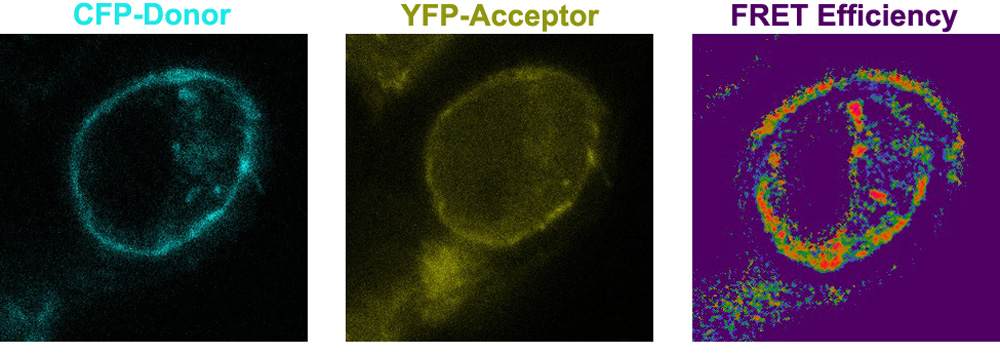
Examining the Role of Tie1 As A Potential Co-Receptor for Tie2 Signaling.
The Tie1 receptor, although highly homologous to Tie2, does not interact with any of the known Angiopoietins and its physiologic ligand(s) are yet to be identified. It is, therefore, classified as an orphan receptor. Although several experiments have recently documented a function for Tie1 in angiopoietin-mediated Tie2 signaling, its role is still poorly defined. Our high-resolution crystal structure of Tie2 has allowed us to develop a homology model for the highly related Tie1. Recent cell-based experiments reported in the literature in conjunction with our model suggest that Tie1 plays a transient role in binding Tie2 during cellular signaling. Specifically, we hypothesis that Tie1 and Tie2 interact through electrostatic interactions mediated by surface side chains. We are therefore assessing the role of Tie1 in Ang/Tie2 complex formation and signaling using a powerful in vivo FRET –based proximity assay.
Biochemical characterization of alpha5beta1 Integrin interactions with Angiopoietin and Tie2.
At the cell surface, Tie2 associates with the primary fibronectin receptor a5b1 integrin allowing extracellular matrix components the capacity to modulate Angiopoietin-mediated signaling. Furthermore, Angiopoietin-1 binds and activates a5b1 in the absence of Tie2 demonstrating that cross-talk between integrins and the Angiopoietins and Tie2 receptor coordinate the regulation of both downstream as well as inside-out signaling pathways. We will use biophysical methods to study the kinetics of the interactions of these important mediators of Angiopoietin-mediated signaling. Specifically, we will identify the regions necessary and sufficient for their interactions as well as estimate the affinities between a5b1 and Ang1 and a5b1 and Tie2 by measuring the equilibrium dissociation constants for each possible interaction. These studies will allow us to begin to appreciate the molecular basis for Integrin-mediated enhanced growth factor signaling.
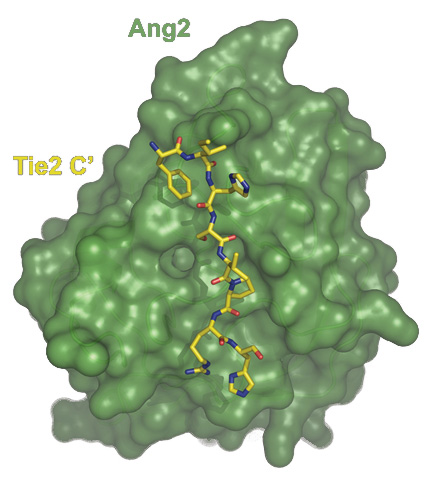
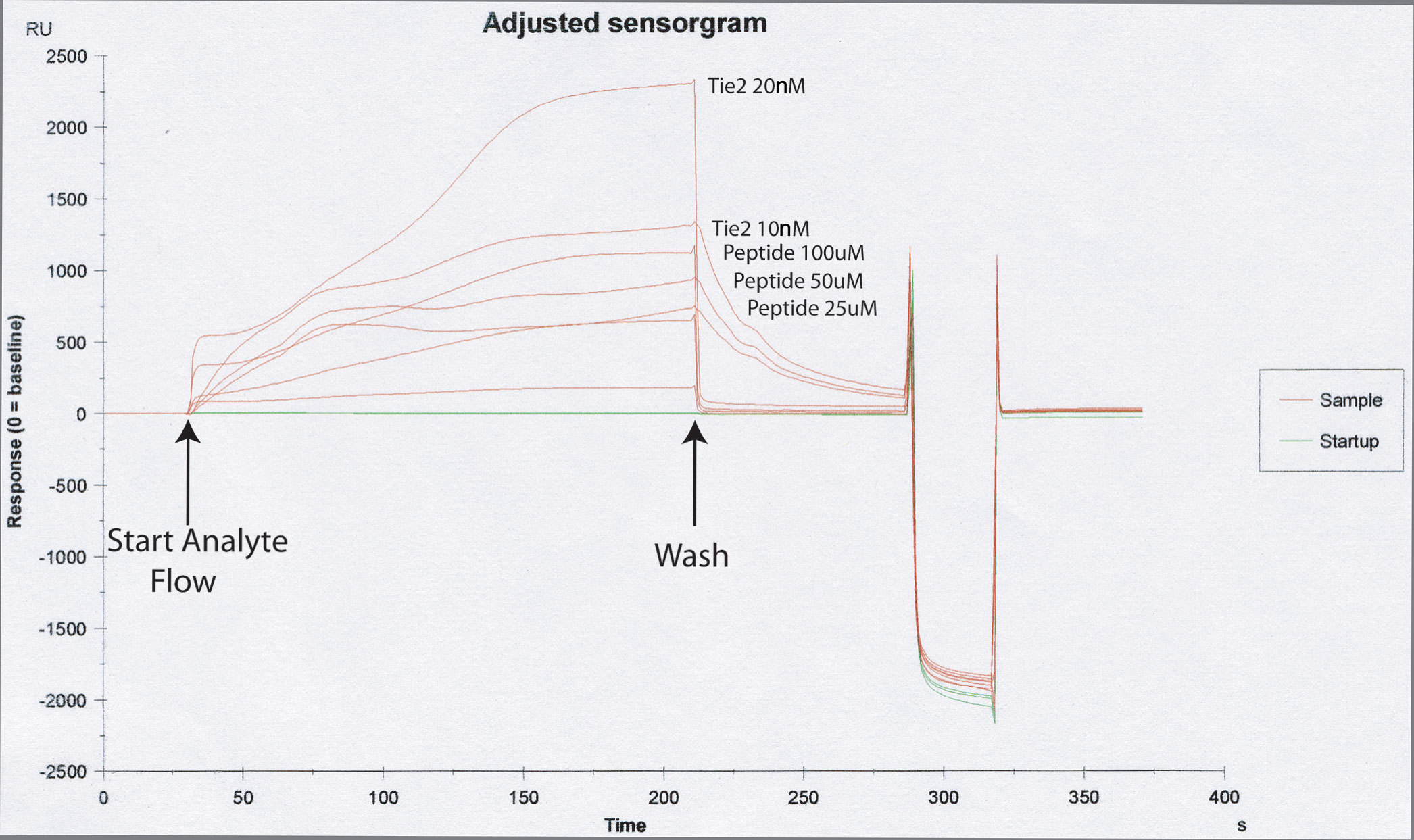
Development of chemical tools for modulating Angiopoietin signaling.
Angiopoietin signaling occurs not only in early development, but continues in the adult organism. Its activation during tumor growth, development, and metastasis suggests that the ability to modulate the receptor-ligand interactions would have important medical applications. Furthermore, our high-resolution crystal structure combined with our mutagenesis experiments indicate that, unlike most other protein-protein interfaces, the Ang2/Tie2 interactions could be effectively disrupted by small peptides. We will therefore use our Ang2/Tie2 complex crystal structure in combination with mRNA display techniques to design peptides that recognize and inhibit the Ang2/Tie2 association. In addition to their potential anti-tumor role, such compounds would be useful for further in vivo studies aimed at dissecting Angiopoietin-Tie2 signaling.