Estuarine Anoxic
Zone
|
| (Life
in
the Dead Zone: Microbial
respiration, production, diversity and gene expression in seasonally
anoxic estuarine waters (NSF-OCE 0961920), P.I.s: Byron C. Crump,
Jeffrey C. Cornwell, Ian Hewson)
|
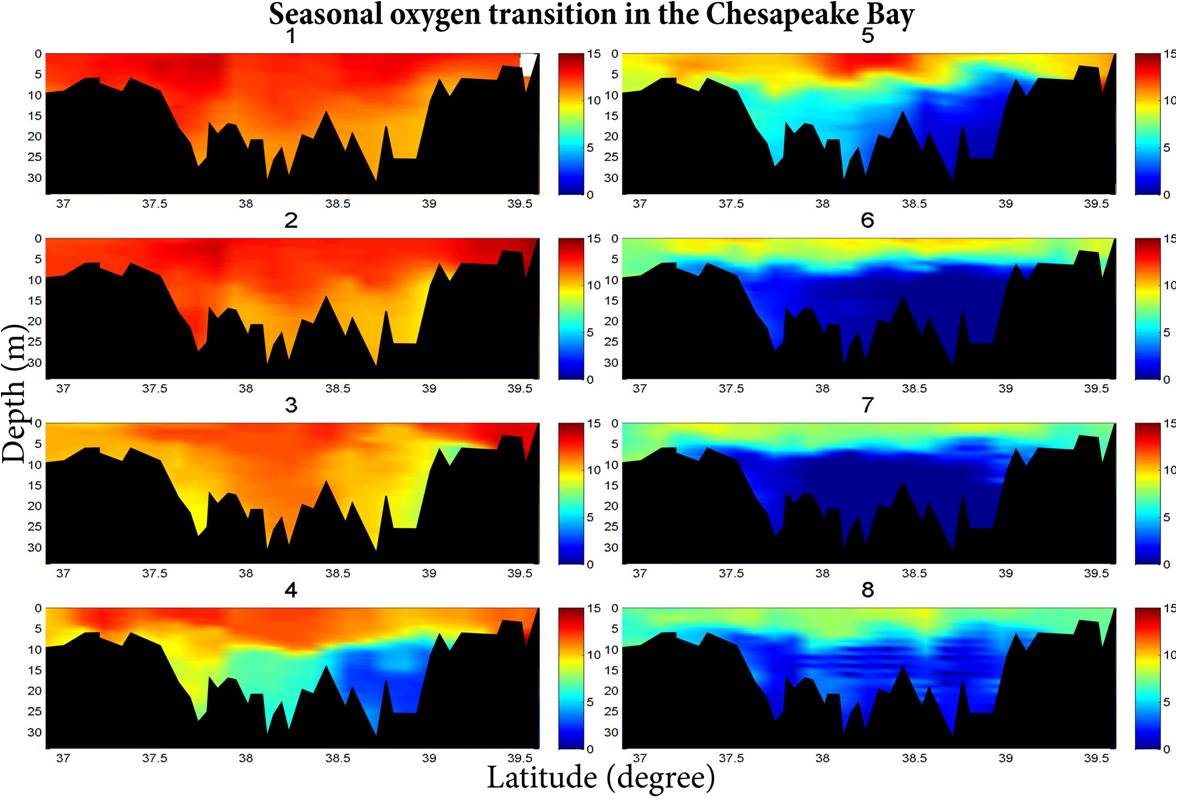
| Figure
1. Seasonal oxygen (mg/l) depletion in Chesapeake Bay. Data,
collected by
the Chesapeake Bay Program, were used to plot oxygen transitions in
2011.
The number on the top of each figure indicates month (e.g., 1 =
January). The black area shows bottom topography and dark blue shows anoxic
regions
covering deep regions of the bay for 4-5 months.
|
|
| Seasonal
oxygen
depletion is a common feature of eutrophic ecosystems. In a temperate
climate zone, the spring freshet caused by melting ice and high
precipitation results in high loads of inorganic
and organic nutrients into aquatic ecosystems. In early
spring, temperature and salinity differences
between surface and bottom water stratify the estuarine water
column and
create a density gradient called a pycnocline. Then, a
composite influence of the
environmental changes and microbial respiration in the water column and
benthic
sediment oxygen demand drive the system from hypoxic to anoxic. |
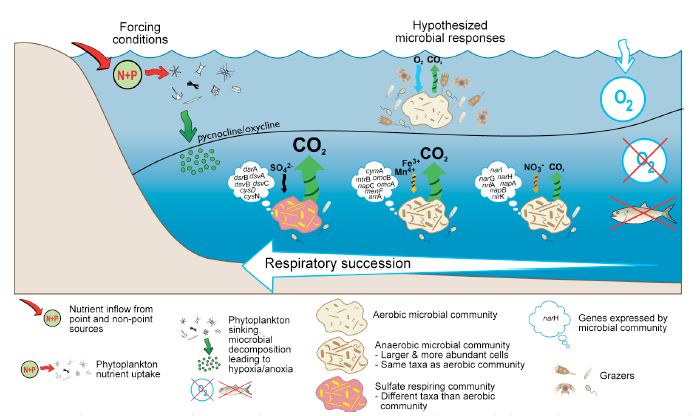
| Fig. 2 Conceptual diagram depicting
respiratory succession in anoxic waters of a stratified estuary.
Reduced growth efficiency
and elevated biomass cause bacterioplankton in anoxic waters to respire
organic matter at a very high rate (Picture courtesy of Dr. Crump). |
|
|
| Once
the
water column becomes anoxic, anaerobes initiate a succession of
respiratory processes that require a sequence of terminal electron
acceptors (Fig. 2). However, large uncertainties are still remain in
the anaerobic metabolism budget in seasonally anoxic environments despite its
importance for estimating ecosystem energy budgets. For this
reason, I have been conducting fine-scale sampling throughout the water
column and sediments from April to October to
determine the influence of rapidly changing environmental and redox
conditions on aerobic and anaerobic respiratory pathways.
|
|
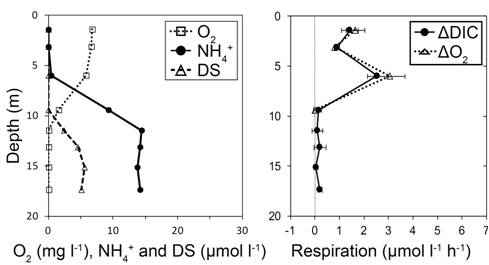
| Fig.
3 Concentrations (left) and community respiration (right) results. DS =
dissolved sulfide, DIC = dissolved inorganic carbon, 'delta' = changes
of DIC and oxygen concentrations indicating respiration rates. |
|
| We found a few interesting points from multiple
measurements shown in Fig. 3. First, we have consistently measured
maximum respiration rates just above pycnoclines (oxyclines) where
oxygen (other reduced nutrients) decreased (increased) most rapidly
towards sediments. Secondly, we have often observed negative
respiration rates, indicating the consumption (chemical reaction) or
fixation (biological reaction) of DIC around and within the pycnocline
(not shown in Fig. 3). From our observations, we hypothesized that
chemoautotrophic and (or) anoxygenic photoautotrophic production (e.g.,
nitrification and sulfur oxidation) may be responsible for
not
only the fixation of DIC but also the increase of total labile organic
matter within and around the pycnocline. To test our hypotheses, we
have been conducting experiments using air-tight bags in anoxic
conditions (Fig. 4) augmented with various electron acceptors and we
indeed measured chemoautotrophic production. |

| Fig.
4 Air-tight water-incubation bags in different sizes for respiration
and chemoautotrophic production measurement. These bags are inserted
into glove bags filled with 99% nitrogen gas. All are incubated in
temperature-controlled chambers. |
|
| As
soon as I measured chemoautotrophic production in the water column, I
realized that we have been missing (or ignoring) the production budget
in estuarine budget calculations. I believe that it is essential to
have finer-scale sampling devices instead of 2-meter interval sampling
devices such as NISKIN rosette bottles or submersible pumps. So far, I
have developed two samplers and they have been used to collect samples every 40cm
within pycnoclines(Fig. 5 and 6). |
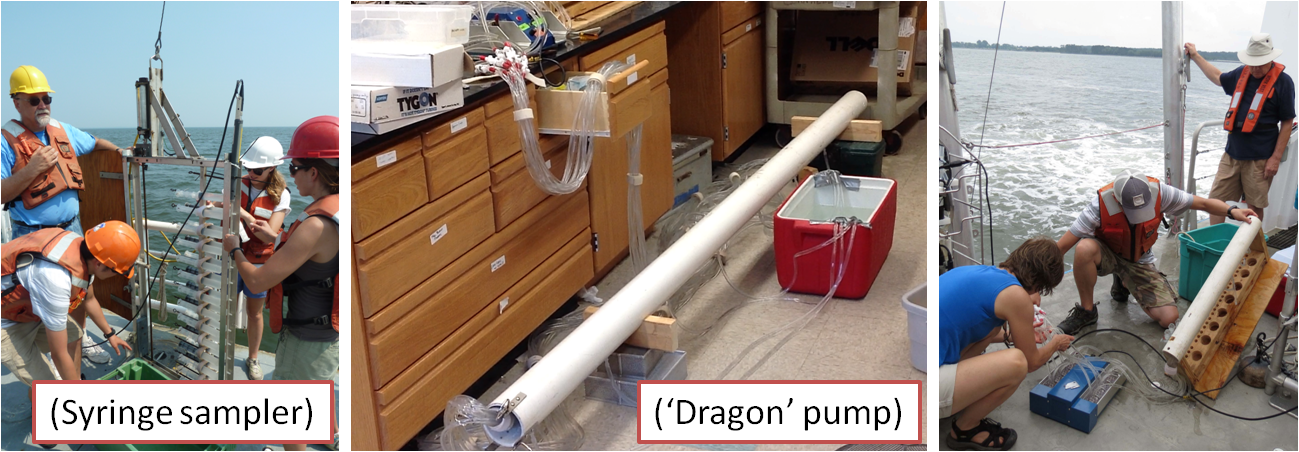
| Fig. 5 Fine-scale sampling device using
either multiple syringes (Syringe sampler) or peristaltic pump
('Dragon' pump) |
|
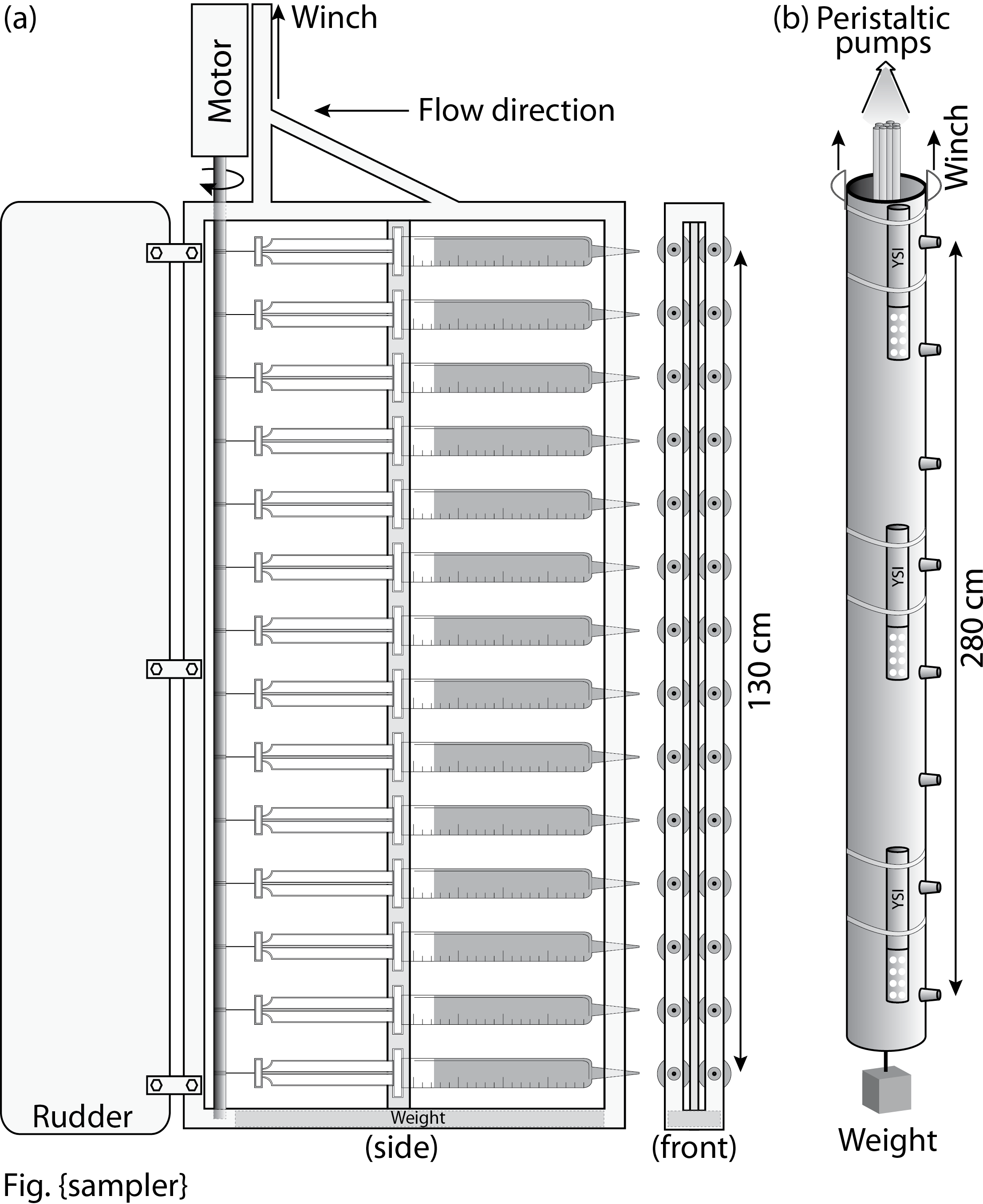 | Fig.
6 Schematic diagram of fine scale sampling devices: (a) syringe sampling
device, (b) peristaltic pump sampling device. The former had been used
in 2011 on the R/V Sharp along with typical water coulmn sampling using
NISKIN bottom collection, but due to its heavy weight and limited volume
of syringes we could not collect 'enough' samples for multiple
analyses. Peristaltic pumping device (aka 'Dragon') has been used for
the same purpose but with technical improvements: x2 length, continuous
sampling, light weight, 3 YSI sensors. |
|
|
|

