The monolayer sample was placed in a solvent that contains phosphors like sodium iodide and zinc sulfide, as shown in the image as a sodium iodide crystal. The phosphors give off light pulses when radiation reaches the sample and a photocathode converts the photons into electrons. The electrons travel through a photomultiplier tube in which they bounce from one dynode (additional electrode) to another. When an electron reaches a dynode, it multiplies and reaches the next dynode in the tube due to the angle at which the dynodes are placed. It keeps multiplying and may reach high amounts like a million electrons depending on how many dynodes are present. After the last dynode, the electric signal is transmitted to some sort of computer device that will process it to measure.
Wijesinghe, D. S., Brentnall, M., Mietla, J. A., Hoeferlin, L. A., Diegelmann, R. F., Boise, L. H., & Chalfant, C. E. (2014)
Ceramide kinase is required for a normal eicosanoid response and the
subsequent orderly migration of fibroblasts
Journal of Lipid Research,55(7), 1298-1309
Ceramide kinase is required for a normal eicosanoid response and the
subsequent orderly migration of fibroblasts
Journal of Lipid Research,55(7), 1298-1309
(Translated by Jeetika Sainani)
Support: Scintillation Counter
|
The scintillation counter was used in the experiment in which arachidonic acid release by fibroblasts was measured. The arachidonic acid was counted by the use of trituum-3, a radioactive compound and isotope of hydrogen. As explained in the experiment section, the arachidonic acid was bought labeled with the trituum-3 already. A monolayer of fibroblasts was incubated with this radioactive compound so that the AA could travel and become incorporated into the membranes of some fibroblasts. The monolayer was rinsed off to remove the unbound arachidonic acid, and it was scratched by a pipette to induce mechanical trauma as a model for a wound in the body.
|
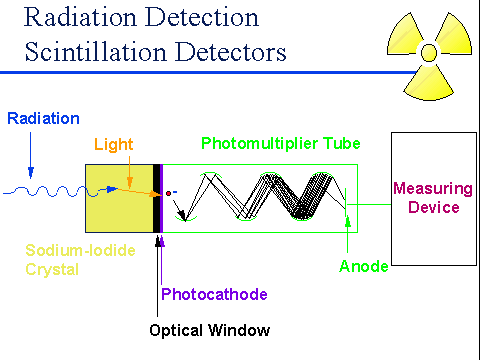
Scintillation Counter Diagram
From http://www.ntanet.net/how-do-sodium-iodide-scintillation-detectors-work
|