Angiopoietin - Tie Signaling in Angiogenesis
The human vasculature is an elegant yet dynamic network of vessels, which serves many vital physiological functions, though perhaps none more essential than delivery of oxygen and nutrients to virtually every cell in the body! As such an essential component of the human body, it should come as no surprise that it plays a crucial role in the development of numerous pathological conditions ranging from cardiovascular disease, to macular degeneration, stroke, and tumor growth and metastasis (to name but a few!). Thus, research focused on evolving a fundamental understanding of this highly dynamic and critical system will have broad implications for human health.
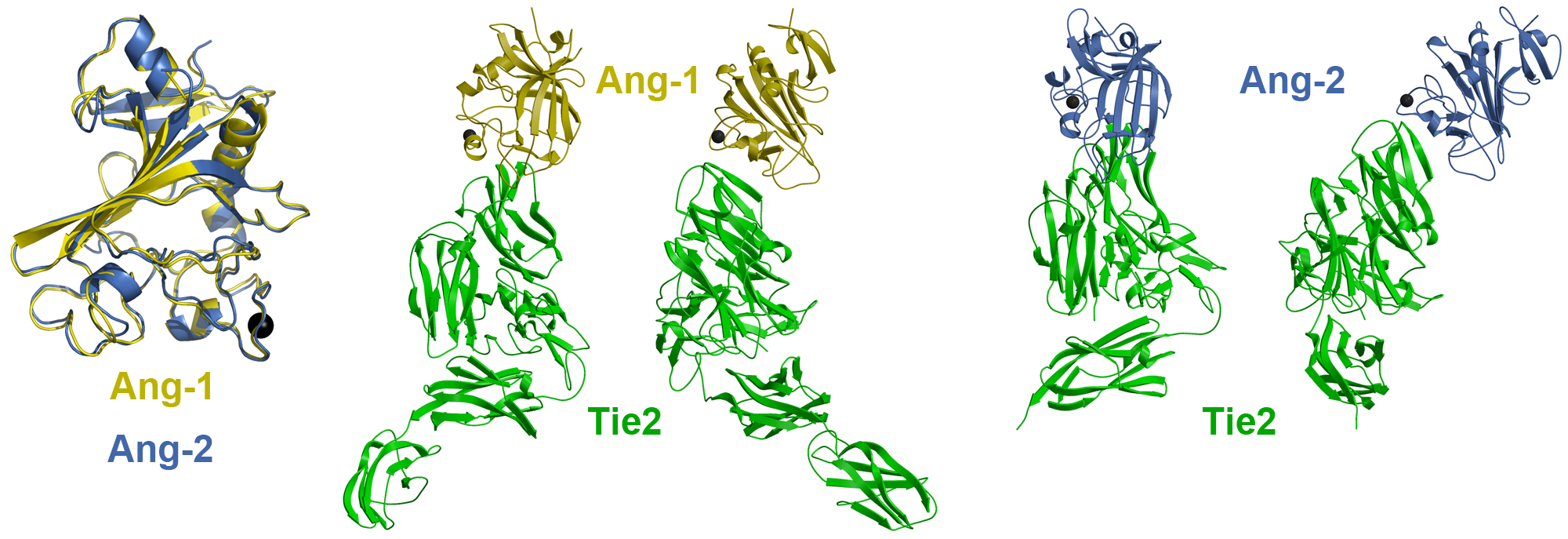
Vascular research has generally focused on the well-established VEGF/VEGFR receptor-ligand system. However, exciting new studies suggest that the downstream Angiopoietin-Tie receptor-ligand system may be more clinically relevant and possess greater physiological functions in vascular homeostasis than previously appreciated. Indeed, the Tie receptor tyrosine kinases and their angiopoietin ligands play central roles in developmental and tumor-induced angiogenesis through their ability to modulate endothelial cell function. In spite of our considerable knowledge of Tie2 signaling, it has only recently been appreciated that Tie2 activity is spatially and temporally fine-tuned through its interaction with functionally related co-receptors including; Tie1 and the integrin cell adhesion receptors α5β1 and αvβ3. Integrin and Tie signaling components coordinate endothelial cell survival, adhesion, and migration in response to the extracellular matrix through complex protein-protein interactions, which have yet to be explored. To develop a better understanding of Tie2 regulation and Tie-integrin receptor cross-talk, we will characterize key interactions between Integrin and Tie signaling components using a combination of complementary structural and functional techniques. Our results will clarify how dynamics in protein-protein interactions on the cell surface between functionally related transmembrane receptors modulate and coordinate Tie2 and Integrin signaling cascades. Moreover, our findings will highlight specific macromolecular structures and cellular functions that may be exploited for therapeutic intervention.