Sulfonyl ureas
|
Sections |
| Structure |
| Structure - Activity Relationship |
| Metabolism and Pharmacokinetics |
| Biochemical Mechanism of Action |
| Questionnaire |
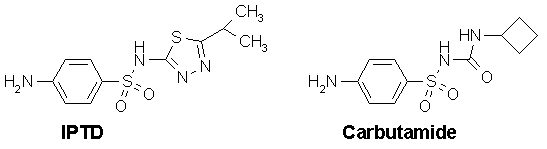
The compound 2-(p-aminobenzenesulfonamido)-5-isopropyl -thiadiazole (IPTD) was used in the treatment of typhoid fever in the early 1940s. However many patients died from obscure causes while being treated with heavy doses of the drug. These deaths were eventually attributed to acute and prolonged hypoglycemia.
IPTD did not come to be used as hypoglycemic agents because a second drug carbutamide was found to an effective oral hypoglycemic agent. Carbutamide was more active than IPTD and was the first sulfonylurea hypoglycemic agent to be marketed.

Todate more than 12000 sulfonyl ureas have been prepared and many have been found to be extremely useful. Six sulfonylureas are currently available in the US. (structures below)

Tolbutamide, Chlorpropamide, Tolazamide and Acetohexamide are first generation sulfonylureas while Glyburide and Glipizide are second generation. (Click on the hyperlinks to view the 3D structures.)
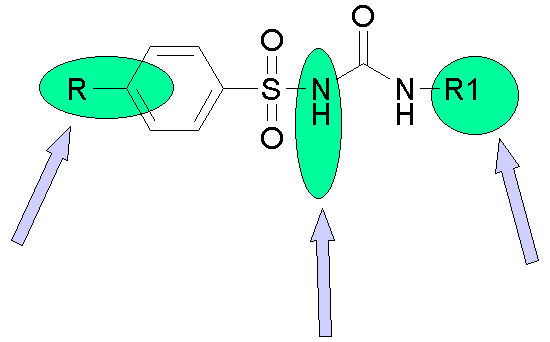
Structure - Activity Relationships
Metabolism and Pharmacokinetics
The sulfonyl ureas are rapidly absorbed from the GI tract. This is understandable taking into consideration their highly hydrophobic nature. Due to this nature they are highly protein bound. Second generation sulfonyl ureas due to their greater hydrophobic character are effective over a longer duration of action.
| Agent | Equivalent
therapeutic dose (mg) |
Serum protein binding (%) | Half-life (h) |
Duration of action (h) |
Mode of metabolism |
| Tolbutamide | 1000 | 95 - 97 | 4.5-6.5 | 6-12 | carboxylation |
| Chlorpropamide | 250 | 88 - 96 | 36 | ~60 | cleavage, hydroxylation |
| Tolazamide | 250 | 94 | 7 | 12-14 | carboxylation, hydroxylation |
| Acetohexamide | 500 | 65 - 88 | 6-8 | 12 - 18 | reduction, hydroxylation |
| Glyburide | 5 | 99 | 1.5-3.0 | ~24 | hydroxylation |
| Glipizide | 5 | 92 - 97 | 4 | ~24 | hydroxylation |
Chlorpropamide is active over an extended period of time because chlorine atom suppresses the rate of metabolism. Despite report to the contrary chlorpropamide does undergo significant metabolism, only slowly. The primary metabolites are 2-hydroxy and 3-hydroxy chlorpropamide.
Hydroxylation of the aromatic ring appears to be the most favored metabolic pathway for these sulfonyl ureas. The hydroxylated derivatives have much lower hypoglycemic activity than the parent compounds.
The alkyl group of the sulfonyl urea also undergoes hydroxylation. For example, Glipizide is metabolized to cis-3-hydroxy-glipizide and trans-4-hydroxy-glipizide. These metabolites have ~15% of the hypoglycemic activity of the parent compound.
Biochemical Mechanism of Action
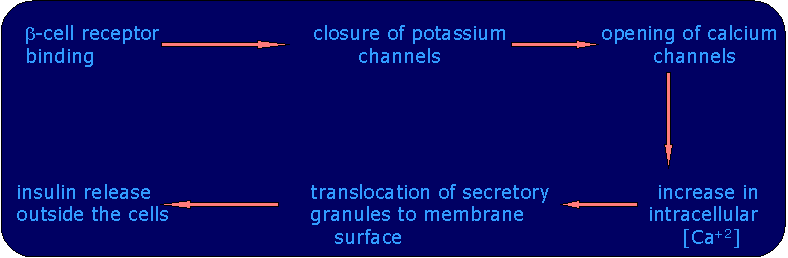
The hypoglycemic action of the sulfonyl ureas is usually attributed to their ability to stimulate the release of insulin from the pancreatic islets. It is proposed that the sulfonyl ureas bring about their increase in insulin release through binding to receptors on the islets b-cell membrane that are linked to closure of the channels that facilitate the passive efflux of K+ from the cell. These K+ channels are responsive to ATP / ADP ratio and close when the ratio increases because of an increase in glucose metabolism. Binding of sulfonyl ureas to their receptor leads to the closure of the potassium channels which opens calcium channels for influx of Ca+2 ions into the cytoplasm. The increase in cytosolic Ca+2 activates the effector system that leads to the translocation of the secretory granules to the exocytotic sites at the plasma membrane at which insulin is released. Thus sulfonyl ureas are effective only if the secretion of insulin from the b-cells not completely impaired but is inefficient.
[top] [session home] [home] [school
of pharmacy] [department of medicinal
chemistry]
©2000 VCU School of Pharmacy
Revised: January 6, 2000
Questions or Comments : Dr. Umesh
R. Desai