Miscellaneous Anticoagulants
| Sections |
| Citric Acid |
Citric acid is a water-soluble tricarboxylic acid widely distributed in plants and in animal tissues and fluids. Lemon juice for example contains 5 - 8 % citric acid. Medicinally, citric acid is used as sodium citrate. When the drug is taken orally the acid is metabolized to bicarbonate anion, which ultimately produces systemic alkalinization for treatment of acidosis. This dose approximately 1 g.
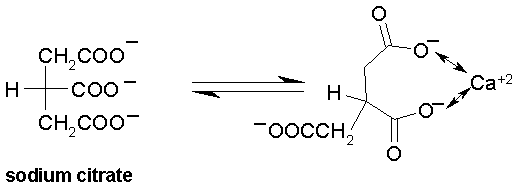
Sodium citrate is an anticoagulant in vitro. The citrate anion sequesters Ca+2 ions and solubilizes them. As repeated mentioned earlier Ca+2 is required for the conversion of several zymogens, e.g., prothrombin, to active proteases, e.g., thrombin. Inavailability of Ca+2 renders the coagulation cascade ineffective.
Sodium citrate cannot be used in vivo because of the toxic manifestations of sequestering Ca+2 ions.
[session home] [home] [department of medicinal chemistry] [school of pharmacy]
©2000 VCU School of Pharmacy
Revised: January 5, 2000
Questions or Comments : Dr. Umesh R. Desai