Heparins
| Sections |
| Structure |
| Structure - Activity Relationships |
| Metabolism |
| Pharmacology |
| Biochemical Mechanism of Action |
| Low-Molecular-Weight Heparins |
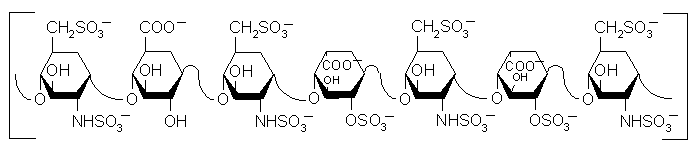
Heparin is a mucopolysaccharide with a molecular weight ranging from 6,000 to 40,000 Da. The average molecular of most commercial heparin preparations is in the range of 12,000 - 15,000. The polymeric chain is composed of repeating disaccharide unit of D-glucosamine and uronic acid linked by 1¯¯>4 interglycosidic bond. The uronic acid residue could be either D-glucuronic acid or L-iduronic acid. (Structure below) Few hydroxyl groups on each of these monosaccharide residues may be sulfated giving rise to a polymer with that is highly negatively charged. The average negative charge of individual saccharide residues is about 2.3.

Structure - Activity Relationship
The key structural unit of heparin is a unique pentasaccharide sequence (below). This sequence consists of three D-glucosamine and two uronic acid residues. The central D-glucosamine residue contains a unique 3-O-sulfate moiety that is rare outside of this sequence. Four sulfate groups on the D-glucosamines, encircled in the figure below, are found to be critical for retaining high anticoagulant activity. Elimination of any one of them results in a dramatic reduction in the anticoagulant activity. Removal of the unique 3-O-sulate group results in complete loss of the anticoagulant activity. Removal of sulfate groups other than the critical ones seems to not affect the anticoagulant activity.
Because of its highly acidic sulfate groups, heparin exits as the anion at physiologic pH and is usually administered as the sodium salt.Heparin is partially metabolized in the liver by heparinase to uroheparin, which has only slight antithrombin activity. Twenty to fifty percent is excreted unchanged. The heparin polysaccharide chain is degraded in the gastric acid and must therefore be administered intravenously or subcutaneously. Heparin should not be given intramuscularly because of the danger of hematoma formation.
Heparin is relatively non-toxic. However, parenteral administration precludes its long-term use. It is generally given to postoperative patients and to those with acute infarctions requiring immediate anticoagulant action.
Heparin overdose or hypersensitivity may result in excessive bleeding. Protamines, highly positively charged low-molecular-weight proteins, are used as anti-dote for excessive bleeding complications.
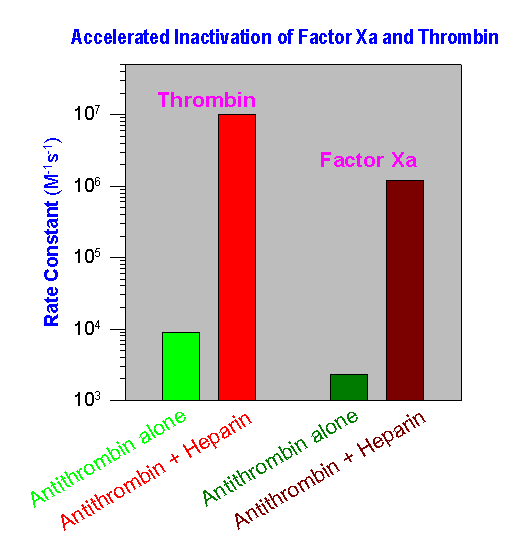
Heparin, containing the unique five-residue sequence (shown above), forms a high-affinity complex with antithrombin. The formation of antithrombin - heparin complex greatly increases the rate of inhibition of two principle procoagulant proteases, factor Xa and thrombin. The normally slow rate of inhibition of both these enzymes (~ 103 - 104 M-1s-1) by antithrombin alone (see graph below) is increased about a 1,000-fold by heparin. Accelerated inactivation of both the active forms of proteases prevents the subsequent conversion of fibrinogen to fibrin that is crucial for clot formation.
Low-Molecular-Weight (LMW) Heparin
As the name implies low-molecular-weight heparins are preparations that have lower average molecular weight than heparin. The average molecular weight of these LMW heparins typically ranges from 2,000 to 8,000 Da. They are made by enzymatic or chemical controlled hydrolysis of unfractionated heparin. These molecules have very similar chemical structure as unfractionated heparin except for some changes that may have been introduced due to the enzymatic or chemical treatment. The mechanism of action of these drugs is the same as full-length heparin.
The overall advantage in the use of these LMW heparins appears to be in the decreased need for monitoring patients in comparison to heparin. Differences of opinion exist and further testing will determine whether these will continue to be used. The first LMW heparin, enoxaparin, has been approved for preventing blood clots following hip replacement surgery.
[top] [session home] [home] [department of medicinal chemistry] [school of pharmacy]
©2000 VCU School of Pharmacy
Revised: January 5, 2000
Questions or Comments : Dr. Umesh R. Desai