Hot, dense materials emit continuous spectra.
Hot, rarefied gases yield emission spectra.
Hot, thin hydrogen gas emits a characteristic set of wavelengths.

In the visible spectrum these wavelengths are: 656 nm (red), 486 nm (blue), 434 nm (violet), 410 nm (violet)
Each element emits a characteristic set of wavelengths.When light with a continuous spectrum passes through a cold, rarefied gas, an absorbtion spectrum results.
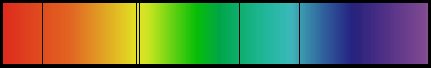
Each gas absorbs the same wavelengths that it emits when it is hot.
The spectrum of the light from our Sun is an absorbtion spectrum.