G-proteins and G-protein coupled receptors:
Guanine nucleotide binding proteins (G-proteins) and their associated G-protein coupled receptors (GPCR's) date back over 1.5 billion years, and have become one of the major mechanisms of signaling in eukaryotes. There are over 500 members of the family of GPCR's, believed to have descended from a common ancestral protein. All GPCR's share a basic structure with seven transmembrane helical domains (hence the name 7-TM proteins). The ligand normally binds in the cleft formed by domains 3,4,5, and 6 (see picture).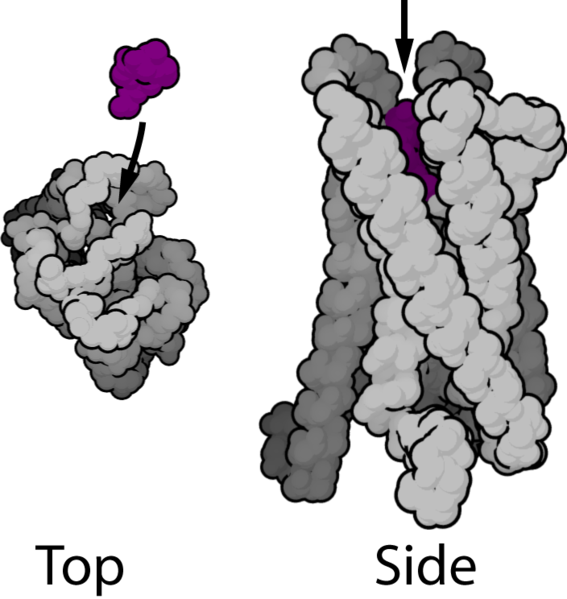 Upon binding the ligand the GPCR's helices shift position so that the cytoplasmic loops can act as catalysts to swap GTP
for a bound GDP in the α unit of the bound G-protein complex.
G-protein complexes have three subunits. The α subunit usually does most of the work;
the β and γ subunits usually bind the α unit to the GPCR, although in some cases they also effect changes on channels when released.
Upon binding ATP the α units release from the β and γ subunits;
most types of α units act in one of the following ways.
Upon binding the ligand the GPCR's helices shift position so that the cytoplasmic loops can act as catalysts to swap GTP
for a bound GDP in the α unit of the bound G-protein complex.
G-protein complexes have three subunits. The α subunit usually does most of the work;
the β and γ subunits usually bind the α unit to the GPCR, although in some cases they also effect changes on channels when released.
Upon binding ATP the α units release from the β and γ subunits;
most types of α units act in one of the following ways.
- Gα s proteins activate one of the Adenylyl Cyclases (ADCY), which generates cyclic AMP from ATP. Cyclic AMP in turn activates another membrane-associated enzyme: protein kinase A (PKA). PKA then phosphorylates a number of other proteins in or near the membrane, including some K+ channels; these close when phosphorylated, leaving the membrane more labile to depolarization. In many epithelial tissues PKA activates proteins that transport the working membrane proteins of that tissue toward the apex of the cell, where they do their work. The effects of PKA on neurons are still being worked out.
- Gα i proteins inhibits Adenyl Cyclase and the production of cyclic AMP. These proteins also open K+ channels, which makes the neuron harder to depolarize.
- Gα q/11 stimulates membrane-bound phospholipase C (PLC); activated PLC cleaves PIP2 (a minor membrane phosphoinositol) into inositol tri-phosphate (IP3) and diacylglycerol (DAG). IP3 is water soluble and travels through the cytoplasm and activates protein kinase C (PKC), which in turn phosphorylates many other targets.
Serotonin receptors:
The 5-HT (serotonin) receptors form a family within the lineage of GPCR's (shown below) as well as one ligand gated channel - HTR3A.
Molecular clock evidence suggests this family started about 800Myr ago.
Some members of this family have since evolved specificity for responding to noradrenalin or dopamine.
Almost all family members have been conserved within the mammal lineage since we branched from reptiles.
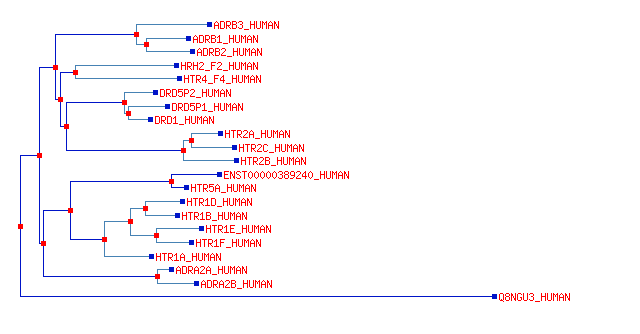
The lower branch (HTR1 family) act by means of Gα i proteins;
these increase K+ conductance and decrease target cell excitability.
The middle branch (HTR2 family) act by means of Gα q/11 proteins and PLC; these decrease excitability.
The upper branch (HTR4 family) acts by means of Gα s proteins, and increases excitability.
The HTR1A receptors are expressed on the cell bodies of the serotonergic cells in the Raphe nucleus and act to decrease serotonin transmission at the synapses.
The D1 family (D1,D5) have short internal loops and long C-tails; the D2 family have long loops and short C-tails.
These physical characteristics predispose D1 family members to recruit Gs proteins, and increase cAMP; they generally decrease K+, and therefore increase the spontaneous activity and also the responsiveness of cells.
On the other hand D2 family receptors primarily recruit Gi proteins and increase K+ conductance, leading to decreased activity.
Our set of 5 distinct dopamine receptors has been conserved from the time of reptiles.
Invertebrates have very distantly related dopamine receptors that don't correspond individually to our receptors.
D1 and D5 are part of the Gs branch of the HTR family (see above).
Several G-protein receptors become active upon binding of peptides with closely paired Tyr/Phe residues at the N-terminal.
These pairs occur at the N-terminal of a number of signaling peptides, including enkephalins,
β-endorphin, and others. Several of these signaling peptides (e.g. β-endorphin) are derived by cutting
fragments from the product of the POMC gene, expressed only in the pituitary gland,
while others (dynorphins) are derived from the PDYN gene, which is expressed in caudate nucleus and pons and less in amygdala,
and others (enkephalins) are derived from the PENK gene, expressed at very high levels in caudate.
The receptors for these peptides are mostly Gs type, which increase cAMP.
Some of these receptors (the μ-opioid receptors) are also triggered by morphine, which led to their discovery.
The receptors come in three main flavors: μ,δ,κ,
which have different distributions in the brain and have acquired different affinities for the signaling proteins.
Most are expressed at modest levels throughout the brain; the δ1 receptor is expressed more in the thalamus.
Oxytocin and vasopressin are nonapeptides, which diverged from a common signaling peptide before the mammalian radiation.
Their receptors are Gq-proteins, which activate PI3K and open K+ channels, thus dampening neuron excitability.
The two receptors diverged also early in vertebrate evolution, and the vasopressin receptor duplicated again in mammals; all three are distributed thinly in the brain.
The Oxytocin gene is expressed abundantly in hypothalamus, and nowhere else, while its receptor (OXTR) is expressed in hypothalamus and sub-thalamic nucleus.
The Vasopressin gene is expressed abundantly in hypothalamus, and nowhere else;
it has two receptors: the AVPR1A receptor is expressed in some smooth muscle and the AVPR1B gene is expressed throughout the brain and in some peripheral tissues.
Corticotropin-releasing hormone (CRH) is a larger (41 aa) peptide, which is also manufactured together with oxytocin and vasopressin in the paraventricular nucleus of the hypothalamus.
Its two receptors (CRHR1 & CRHR2) are highly conserved GPCR's which diverged prior to chordate evolution;
they are distantly related to a diverse class of other GPCR's that respond to other peptides and hormones.
Both CRH receptors act by Gs type, but signaling is complex, because several splice variants of CRHR1 are expressed, and several do not seem to have cAMP-inducing activity.
In some cells there seems to be actions mediated by PLC activity also.
CRHR1 is moderately expressed in cortex and cerebellum.
CRHR2 is moderately expressed in striatum and septum.
Cortisol is a corticosteroid hormone or glucocorticoid produced by the adrenal gland.
The two main cortisol receptors are both nuclear receptors: NR3C1 (glucocorticoid receptor) and NR3C2 (mineralocorticoid receptor).
Nuclear receptors directly change transcription levels: NR3C1 has a broadly anti-inflammatory effect.
These are most closely related to the progesterone receptor; the NR3C receptors diverged from each other 400-500 M yr ago, and have been very highly conserved since.
NR3C1 is expressed at high levels in cortex, thalamus and hippocampus, and in blood.
NR3C2 is expressed at high levels in pre-frontal cortex, and in blood.
Noradrenalin receptors:
There are three families of noradrenaline receptors (ADR's): the α2 and β receptors are sub-clades within the family of HTR receptors shown above.
The α1 receptors are members of a Gα q/11 acting lineage with no close relatives.
Dopamine receptors:
Peptides
The HPA Axis