Radiopharmaceuticals for Heart
Myocardial Imaging Agents
Planar and SPECT Agents |
PET Agents |
Blood pool markers |
Perfusion Agents |
Tc-99m Albumin |
Rb-82 rubidium chloride |
Tc-99m red blood cells |
O-15 water |
Infarct-avid Agents |
N-13 Ammonia |
Tc-99m pyrophosphate |
Metabolism Agents |
In-111 imciromab pentetate |
C-11 acetate |
Perfusion Agents |
C-11 palmitate |
Tl-201 thallous chloride |
F-18 fludeoxyglucose |
Tc-99m sestamibi |
Hypoxia Agents |
Tc-99m tetrofosmin |
F-18 FMISO |
Tc-99m teboroxime |
Sympathetic Nerve Agents |
Tc-99m nitrido dithiocarbamate [Tc-N-( NOEt) 2] |
mIBG |
Perfusion Imaging Agents
- Thallous Chloride Tl 201 Injection
- Compounding
- Thallous chloride Tl 201 injection is a sterile aqueous solution that contains at the time of calibration 1 mCi/ml of Tl-201 chloride in 0.9% sodium chloride solution, pH adjusted to 4.5 to 7.0 and preserved with 0.9% benzyl alcohol.
- Multidose vials in 2, 4, 8, and 9 mCi sizes are available at different calibration times throughout the week. Its radiochemical purity is NLT 95%. At the time of calibration, it contains no more than 1.0% each of Tl-200 and Tl-202 and NMT 0.25% Pb-203 as Radionuclidic impurities, and NLT 98% Tl-201 as the desired radionuclide. The product is stored at room temperature.
- Clinical Use – Myocardial perfusion agent. For Tl-201 Rest/Redistribution protocol, 2 –4 mCi is administered iv at 0 time. For Tl-201 Stress/Redistribution/ Reinjection protocol, 2-4 mCi is administered at maximum dilation of coronary arteries (2-4 mCi) and 1 mCi following redistribution.
- Localization – Tl-201 has a potassium-like distribution (analog) due to similarities that exist between the two elements (monovalent cation charge, similar sized hydrated radii). Thallous ion activates the Na-K-ATPase system. About 4% i.d. localizes in the heart.
- Biodistribution – About 15% of Tl-201 is taken up by the liver and 3.5% by the kidneys. Early lung uptake (8-10%) clears by the time of the redistribution image in normal volunteers. Retained lung activity correlates with pulmonary artery disease.
- Excretion – Urinary excretion accounts for 4-8% of activity in the first 24 h after iv administration, and fecal excretion is insignificant. Tl-201 is excreted in breast milk. Patients who are breast feeding should stop nursing for at least 3 weeks.
- Tc-99m Sestamibi Injection
- Compounding - Tc-99m sestamibi injection is a sterile aqueous solution prepared from a kit consisting of a lyophilized mixture of tetrakis (2-methoxy isobutyl isonitrile) copper (I) tetrafluoroborate (1.0 mg), sodium citrate dihydrate (2.6 mg), L- cysteine HCl monohydrate (1.0 mg), manntol (20 mg), and stannous chloride dihydrate (0.025 – 0.075 mg) sealed under nitrogen.
- The kit is prepared by adding 25 to 150 mCi Tc-99m sodium pertechnetate in 1 – 3 mL, mixing vigorously to dissolve the powder and placing the vial in a boiling water bath for 10 minutes.
- The following reactions take place:
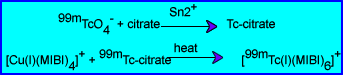
- The vial is allowed to cool 15 m before use. The labeled product is stored at 15 – 25 °C and is stable for 6 h.
- Clinical use – Myocardial perfusion agent.
- Localization
- Tc-99m sestamibi enters the myocardium by passive diffusion because of its lipophilicity and is retained for a prolonged period of time.
- Uptake is related to the integrity of the plasma and mitochondrial membrane potential in myocytes.
- 90% of Tc-99m sestamibi is associated with mitochondria in an energy-dependent manner as a free cation complex.
- The attraction of Tc-99m sestamibi to mitochondria is promoted by a negative potential generated in the mitochondrial membrane of viable myocytes implying it is a marker for viability and perfusion.
- Its uptake is not affected by ouabain. Uptake is 1% i.d. after 60 m at rest and 1.4% after exercise.
- Biodistribution
- Highest activity is achieved in gallbladder and liver, followed by the heart, spleen, and lungs.
- Non-cardiac activity decrease more rapidly with time and more rapidly with exercise than at rest.
- Resting images are best performed at 60 minutes when at rest and may be shortened to 15 – 30 minutes following exercise.
- Following pharmacologic stress images are performed at 45 – 60 minutes to allow for clearance of the splanchnic bed.
- In every situation imaging is based on when there is clearence from the hepatobiliary tree and/or small bowel.
- Excretion
- Tc-99m sestamibi is excreted intact principally by the kidneys and the hepatobiliary system.
- By 24 hrs, urinary excretion is 30% and 24% of the initial dose, after rest and exercise studies, respectively.
- By 48 hrs, fecal excretion is 37% at rest and 29% after exercise.
- Tc-99m Tetrofosmin Injection
- Compounding - Tc-99m tetrofosmin injection is a sterile aqueous solution prepared from a kit containing a lyophilized mixture of tetrofosmin (0.23 mg), stannous chloride dihydrate (30 μg), disodium sulfosalicylate (0.32 mg), sodium D- gluconate (1.0 mg), and sodium bicarbonate (1.8 mg) sealed under nitrogen. The kit is stored at 2 – 8 °C before reconstitution.
- Labeling is achieved by introducing a venting needle to the kit and adding up to 240 mCi of Tc-99m pertechnetate (4 – 8 mL and NMT 30 mCi/ml).
- This is followed by removal of 2 mL of gas from the vial and incubation at RT for 15 m. The following reactions take place:

- The labeled product is stored at 2 – 25 °C after labeling and is stable for 8 hours. The radiochemical purity must be ≥90%.
- Clinical use – Myocardial perfusion agent.
- Localization
- The most likely mechanism is a potential-driven diffusion of the lipophilic cation across the sarcolemmal and mitochondrial membranes.
- Tc-99m tetrofosmin appears to be associated more with cytosol than within the mitochondria.
- Uptake in myocardium is 1.2% i.d. at 1 hr at rest and slightly higher with exercise.
- Biodistribution
- Liver uptake is 2.1% at 1 hr. Heart-to-liver ratio at 1 hr at rest is 1.2 and 3.1 after exercise.
- There is rapid clearance from abdominal organs allowing early imaging.
- Excretion – Approximately 66% of the i.d. is excreted in 48 h, about 40% in urine and 26% in feces.
- Ammonia N-13 Injection
- Compounding - Proton irradiation of O-16 water results in production of N-13 ammonia was well as N-13 nitrate and N-13 nitrite. These forms can be converted to N-13 ammonia by chemical reduction using DeVarda’s alloy in aqueous sodium hydroxide or titanium (III) salts.
- Clinical use – Evaluation of myocardial blood flow (10 – 20 mCi). Since the half-life of N-13 is 10 m, rest and stress studies may be performed 30 minutes apart.
- Localization – Following uptake in the myocardium N-13 ammonia is rapidly fixed as N-13 glutamine by the enzymatic conversion of glutamic acid by glutamine synthetase.
- Biodistribution - First pass myocardial extraction is high (>90%). N-13 ammonia is a lipophilic and uncharged molecule that diffuses rapidly across the capillary endothelium and sarcolemma of the myocyte.
- Rubidium Chloride Rb-82 Injection
- Compounding – Rb-82 chloride is a generator-produced nuclide with a half-life of 75 s. It is the daughter of Sr-82 (T ½ = 25d) and eluted from the generator (useful life of 4 – 6 weeks) with 0.9% sodium chloride injection.
- Clinical use – Perfusion imaging (40 – 60 mCi). Its short half-live allows multiple studies every 10 m.
- Localization – Rb-82 is taken up across the sarcolemmal membrane via the Na-K ATPase pump.
- Biodistribution – First pass myocardial extraction at 1 and 3 mL/min/g tissue is 59% and 26%, respectively.
- Water O-15 Injection
- Compounding – O-15 water can be produced by reduction of O-15 oxygen. Hydrogen gas is admixed with a stream of O-15 oxygen and passed over palladium or platinum catalyst heated to 450 °C The reduced O-15 water is bubbled through sterile water.
- Clinical use – Ideal tracer for perfusion studies (30 mCi).
- Localization – Rapid diffusion
Metabolism Agents
- Fludeoxyglucose F-18 Injection
- Sodium Acetate C-11 Injection
- Compounding – Carbonation of methylmagnesium Grignard reagent with C-11 carbon dioxide, followed by acid hydrolysis to form C-11 acetic acid. The product is purified by solid phase extraction.
- Clinical use – Measurement of myocardial oxidative capacity (overall oxidative metabolism) by analysis of uptake and clearance curves.
- Localization – Actively extracted by the myocardium and activated to acetyl- CoA, which is oxidized in the mitochondria to C-11 carbon dioxide and water.
Blood Pool Imaging Agents
- Tc-99m Red Blood Cells
- In-Vitro Method ( UltraTag RBC- Mallinckrodt)
- Components:10 mL Reaction Vial contains a lyophilized mixture of:
- stannous cloride dihydrate 105 μg
- sodium citrate dihydrate 3.67 mg
- dextrose anhydrous 5.5 mg
- pH 7.1 – 7.2
- Syringe I (0.6 mL)
- sodium hypochlorite 0.6 mg
- pH 11-13
- Syringe II (1.0 mL)
- citric acid monohydrate 8.7 mg
- sodium citrate dihydrate 32.5 mg
- dextrose anhydrous 12 mg
- pH 4.5 – 5.5
- Preparation:
- Collect 1 – 3 mL patient’s blood. Use 0.15 mL ACD or 10 – 15 units heparin per mL blood maximum. Do not use EDTA or oxalate as anticoagulants.
- Transfer blood to reaction vial to dissolve crystals. Incubate for 5 min.
- Add contents of syringe I. Mix by gentle inversion 5 times.
- Add contents of syringe II. Mix by gentle inversion 5 times.
- Add 10 – 100 mCi sodium pertechnetate in up to 3 mL.
- Mix gently 5 times. Incubate 20 min to label cells.
- In-vivo Method
- Reconstitute one vial of TechneScan PYP (Mallinckrodt) that contains about 15.4 mg Sn- PPi (2 mg Sn 2+) with 5 mL saline.
- Inject the patient with 1.4 mg Sn- PPi per 1000 mL whole blood volume. An average adult 70 Kg male (~ 5400 mL whole blood) would receive 7.5 mg Sn- PPi (one-half vial) per dose, equivalent to ~ 15 μg Sn (II)/Kg.
- Within 20 to 30 min of injecting the Sn- PPi, 15 – 25 mCi of Tc-99m sodium pertechnetate is administered intravenously.
- Modified In-vivo Method
- Administer ~ 500 μg stannous ion as Sn- PPi ( Pyrolite) intravenously.
- 20 min later, withdraw 3 mL of tinned RBCs through a heparinized butterfly infusion set into a shielded syringe containing 20 mCi of pertechnetate.
- Incubate the mixture for 10 minutes
Infarct-Avid Imaging Radiopharmaceuticals
- Tc-99m Pyrophosphate (Tc-99m PPi)
- Compounding – A sterile aqueous solution prepared from a kit containing a sterile lyophilized mixture of sodium pyrophosphate (or with a trimetaphosphate )and stannous chloride sealed under nitrogen.
- The kit is prepared by adding the required amount of Tc-99m sodium pertechnetate and allowing the kit to stand for a few minutes.
- The reconstituted product has a pH 4.0 – 7.5 and is stable for 6h.
- The radiochemical purity must be ≥ 90%.
- Clinical use – Bone and cardiac (infarct-avid) imaging (15 mCi).
- Localization
- Ischemic damage to the myocardial cell membrane produces an imbalance in the myocyte’s normal intracellular (10 -7 M)-extracellular (10 -3) Ca ++ concentration.
- Following infarction with disruption of myocyte membrane, calcium ions diffuse and deposit in mitochondria where hydroxyapatite crystals form.
- Tc-99m PPi undergoes sorption to various forms of tissue calcium stores, including amorphous calcium phosphate, crystalline hydroxyapatite, and calcium complexed with various macromolecules at the infarction site.
- Tc-99m PPi begins to localize in infracted tissue within 12 – 24 hours. Scintigrams become more positive during 24 – 72 hours and remain abnormal for 6 days after infarction, fading thereafter and becoming negative by day 14.
- In-111 Imciromab Pentetate (In-111 antimyosin) ( Myoscint – Centocor)
- Localization is based on the disruption of the sarcolemmal membrane of dying myocytes exposing myosin filaments that are normally segregated from extracellular fluid.
- Tc-99m Glucarate
- Localizes in irreparably damaged myocytes where it is associated with highly basic histones.
- Tc-99m Annexin V
- Localizes on exposed phosphatidylserine on apoptotic cells.
Radiotracers for Imaging Hypoxia
Most tracers prepared and evaluated are nitroimidazole based. Nitroimidazoles are a class of lipophilic compounds with high electron affinity. These compounds readily diffuse through cells. The nitro group can be chemically reduced to form a radical, which under normal oxygen content will react to regenerate the parent compound and diffuse out of the cell. Under hypoxic conditions, the nitro radical can interact with intracellular macromolecules and are trapped.
- F-18 fluoromisonidazole (FMISO)
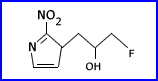
- Uptake of FMISO in ischemic and hypoxic hearts is twice that of normal. Myocardial uptake is proportional to the level of tissue hypoxia. There is increased uptake of FMISO in intact ischemic heart that decreases with progressively longer periods of coronary occlusion. Necrotic myocardium has low uptake.
- FMISO is synthesized by fluorination of O-THP protected tosylate of misonidazole followed by acidic deprotection.
Radiotracers for Myocardial Sympathetic Nerve Imaging
The major neurotransmitter of the sympathetic nervous system is norepinephrine (NE), which is taken up by the transporter from circulation and stored in neuronal vesicles by vesicular monoamine transporter.
- Meta- iodobenzylguanidine (mIBG)
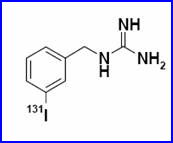
- Compounding – mIBG is labeled either with I-123 (prepared on site) or with I-131, available as a commercially prepared product. I-131-mIBG is supplied as a frozen solution in a concentration of 2.3 mCi/mL. The product should be kept frozen until use and should be used within 6 h after thawing. Labeling yields are typically 98% or higher, requiring no purification step to remove unbound iodide.
- Clinical use – Cardiomyopathy, primary or metastatic pheochromocytomas, neuroblastomas and carcinoids.
- Localization - MIBG shares cellular transport and storage mechanisms with NE. Both MIBG and NE enter neuronal cells through the uptake-1 mechanism and are stored in chromaffin granules and secreted in response to acetylcholine. Sympathetic nerves rapidly take up MIBG. Using sympathetic denervation models, a profound loss of MIBG uptake in the affected myocardium was observed. Similarly, a marked loss of MIBG uptake was observed in patients with heart transplants. Numerous application of MIBG in studying sympathetic innervation and dysfunction of the heart in man have been reported.
Return to the Beginning of the Document
Return to the Table of Content



