- When you extract 99mTc these points should be taken into consideration
- Elute the 99Mo/99mTc generator
- Measure the amount of activity
- Check for Molly breakthrough
- Check for Al+3(colorimetric test)
- Preparing a Technetium radiopharmaceutical
- The elution is a salt Na + 99mTcO4- and the radioactive atom is in a plus seven valence state
- In order to chelate it to another compound, you must first reduce 99mTc atom (exception is sulfur colloid)
- 99mTc has to be reduced to either a +3, +4, or +5 valence state
- Changing the valence depends on the type of reducing agent used
- Depending on the pharmaceutical being, the valence state must vary in order to chelate to a specific compound
- Hence, a specific reducing agent is used pending the specific valence stated required by the pharmaceutical
- As an example, of reducing agents:
- In the presence of SnCl2 or Sn 99mTc is reduced to 5+ valance
- In the presence of Sn + HCl 99mTc is reduced to 4+ valance
- As an example, one could prepare 99mTcMAA
- A specific amount of activity (99mTcO4-) is added to a cold vial of MAA (also referred to as the reaction vial)
- SnCl2 reduces 99mTcO4- to the 4+ or 5+valence state ("No studies have identified the nature of the complexation or the oxidation state of technetium bound to MAA particles.)1
- The reduced 99mTc atoms then chelate to MAA
- Let the reaction vial stand for 15 minute but give it an occasional swirl. It is then ready to be injected into the patient, unless you decide to complete several QC procedures
- Because MAA is insoluble in saline there you will not have to test for 99mTcO2, this by-product is not present (see comments on Hydrolyzed Reduced [HR], below). However, you can test for the percent of MAA bound to the 99mTc
- A closer look at the chelating process
- Reduction and Oxidation of 99mTcO4- (refer to the graph below)
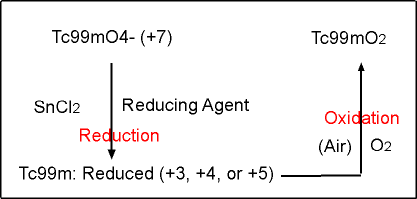
- As previously stated, when 99mTc is eluted out of the generator it is in the 7+ valence state
- The reduction agent causes the 99mTc atom to be reduced, allowing it to chelated to another compound
- Most cold kits are in a nitrogen-rich environment (lacking O2) and usually the compound is lypophilized
- Sn reduces the pertechnetate which allows it to chelate
- However, if air is added into the vial, O2 will cause oxidation of the 99mTc atom
- The oxidation of the 99mTc atoms causes the valence state to return to 7+
- 99mTc and the compound it is tagged to breaks down
- It becomes Hydrolyzed Reduced (HR) (aka known as 99mTcO2 (technetium dioxide)
- This process only occurs with radiopharmaceuticals that are water soluble (most of the agents used in NMT are water soluble)
- Oxidation causes free technetium to be administered to the patient
- Where would free technetium go?
- Other factors that result in a loss of radiochemical purity
- Sterile saline that has any bacteriostatic agents may interfere with the tagging process. Therefore, use preservative free sterile saline
- What happens if there is moisture in the reaction vial and SnCl2 is used as a reducing agent?
- SnCl2
becomes hydrolyzed
- Colloid forms
- Liver/spleen uptake would be noted if injected into a patient
- TLC - Defining radiochemical purity
- Applying Thin Layer Chromatography (TLC)
- How does TLC work?
- Chromatography is a process in which a liquid can be separated into its individual components, via its solubility to certain solvents. In nuclear medicine, certain solvents separate the different components in a radiopharmaceutical as a solution climbs up a strip of "paper" via capillary action. See process below:
- 99mTcMAA, a non-water soluble radiopharmaceutical is going to be tested for radiochemical purity
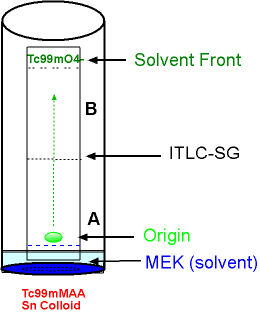
- Silica gel strips impregnated in glass fiber (ITLC-SG) is the media
- In the diagram, MEK is the solvent
- The green drop of the prepared 99mTc compound is placed on the ITLC-SG strip
- Components in the radiopharmaceutical are identified: 99mTcMAA, 99mTcO4-, and Sn Colloid.
- The strip is put into the MEK solution (note that the radiopharmaceutical drop needs to be above the solution)
- The components of the radiopharmaceutical separate along the solvent front, and free technetium (99mTcO4-) migrates to the top of strip
- The remaining components are not soluble in MEK and they remain at the origin
- An example of a water soluble radiopharmaceutical would be 99mTcMDP. Here is an assessment on how to determine its radiochemical purity
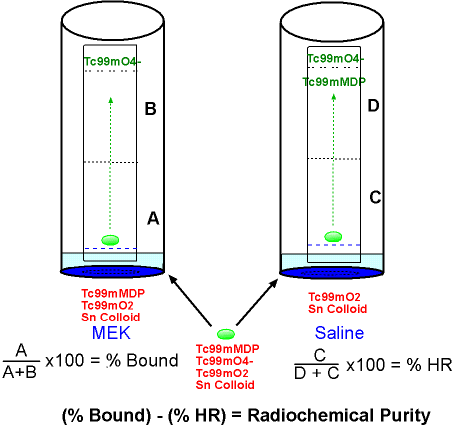
- In this diagram MEK and saline are the two solvents
- The green drops are the prepared 99mTc compound which is placed on ITLC-SG strips
- Components of the radiopharmaceutical that are present include: 99mTcMDP, 99mTcO2, 99mTcO4-, and Sn Colloid.
- One strip is put into the MEK solution and the other strip is put into a saline solution (note that the radiopharmaceutical drop needs to be above the solution)
- The components in the radiopharmaceutical separate as the solvent front migrates to the top of strip
- In the MEK solution, free technetium (99mTcO4-) is soluble which results in its migration with the solvent front to the top of strip
- In the saline solution, 99mTcMDP and 99mTcO4- migrate with the solvent front to the end of strip
- Calculating radiochemical purity can now be determined
- The strips are then taken out of the vial and each strip is cut in two pieces (note the dotted line in the middle of both strips)
- Strips are labeled A, B, C, and D
- Strips are usually assessed with either a well counter or a dose calibrator
- Formulas are applied
- Radiochemical purity is determined
- Let's check our numbers!
- A = 147290, B = 2195, C = 3419, D = 214609, and Bkg = 49
- A = 147290 - 49 = 147241, B = 2195 - 49 = 2146, C = 3419 - 49 = 3370 and D = 214609 - 49 = 217930
- % Bound = 149387/151533 = 98.7%
- % HR = 3370/214560 = 1.6%
- Radiochemical purity = 98.7% - 1.6% = 97.1%
- Ranges for radiochemical purity
- UPS's lower limit for sulfur colloid is 92%
- UPS's lower limit for most other technetium-based compound is 90%
- QC on the MAA particles
- MAA particles have an acceptable range
- 10 to 90 microns at 90%
- 91 to 150 microns less than 10%
- Determining the size of a particle
- Use a hemocytometer and a microscope
- The hemocytometer is a device normally used to determine the size and amount of RBCs and WBCs in a patient's blood
- Hemocytometer may also be used to determine MAA particle size (see diagram below)
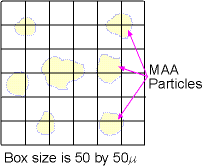
- Place a drop of 99mTcMAA onto the slide
- Put hemocytometer slide over the drop, multiple size grids will be seen
- Assume in the example above the grid is 50μ x 50μ)
- Put the hemocytometer under a microscope
- Determine particle size by matching the particles to the 50x50 micron grid
- What is the approximate size of the smallest/largest particle?
- Does it the size criteria?
- When looking at the size of sulfur colloid with this method may also be employed
- The largest acceptable particle is 1.0μ
- A light-based microscope can only resolve down to 2.0μ
- So, if you see any particles, they are too BIG!
Return to the beginning of the document
Return to the Table of Contents
10/23
1. http://pharmacyce.unm.edu/nuclear_program/freelessonfiles/Vol12Lesson3.pdf"VOLUME 12, LESSON 3 - Technetium Radiopharmaceutical Chemistry" by RJKowalsky - Link



