- Stable bond between shared elements
- Usually is a covalent bond
- What is a covalent bond?
- Covalent bonds tend to be more stable in various environments
- Key - Filling the electron shells (8)
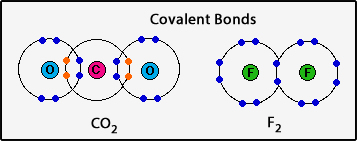 1
1
- This process will altering the biological (or) structure of a labeled compound
- Denaturing of a protein can be done with
- Heat
- Chemical
- A pH (below 2 or above 10)
- What procedure uses denaturing prior to injecting the radiopharmaceutical?
- Different mass number of the same isotope cause alteration in weight that may change the effect on how a isotope will bind
- The difference between H and 3H is an example where the effect can be seen. 3H as part of water which behave differently when compared to 1H in water. Tritium Oxide is also know as heavy water, is radioactive, and has its roots in the the making of atomic bomb material
- However, Isotopes with larger mass numbers tend not to have this problem (123I and 131I)
- So it depends"heavy" the atom is
- When an radiopharmaceutical is carrier free it may mean one of two things
- There is only one radionuclide or isotope in the compound
- And/or another isotope has not been added into solution
- Rational for adding another isotope (usually a stable isotope) is to prevent the agent from adhere to container wall
- Inappropriate storage will cause decomposition of the compound
- Depending on the type of radio-agent it can be sensitive to: light, temperature, and/or radiolysis
- Why do a lot of medication bottles composed of dark tainted glass?
- What type of emitter might have a greater radiolysis issue?
- Activity per gram - usually we want high specific activity
- However, in some cases there might cause too much gamma and/or beta radiation being emitted, which could effect the chemical bond by causing radiolysis
- If a radio-labeled compound breaks down by the emitted particles or rays this is known as autoradiolysis
- If the radiation causes the production of free radicals then this is known as indirect radiolysis. Indirect radiolysis with water
- The two factors that must be considered in a radioactive compound are: T1/2 and how energetic the emissions are
- When comparing fission products to cyclotrons products there tends to be a greater amount of impurities in fission process. This is because a large atom decays to many smaller ones
- Chemical separation must be completed to remove contaminates
- But what happens if you want to chemically separate 123I from 124I, where 124I is the contaminant. Can you remove the impurity?
- Sometimes the unwanted ingredient has a shorter T1/2 which can be separated from solution or decay out of solution
- 203Tl(p,3n)201Pb (T1/2 = 8.4 hrs)
- 201Pb decays to 201Tl (T1/2 = 73.1 hrs)
- Radiolysis or physical T1/2 the life a product will affect its shelf-life
- Three physical T1/2 or 6-months is usually the products limitation
Return to the Table of Content