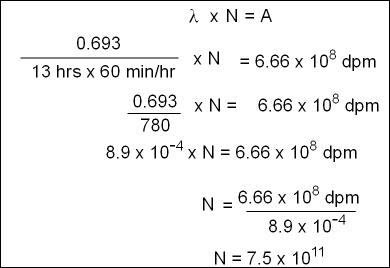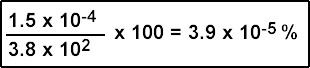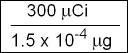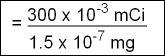- Fission is a process in which one creates smaller (radio) nuclides from larger (radio) nuclides
- Splitting a heavy nuclei releases energy and creates smaller fragments or nuclei in the process
- Fission in an uncontrolled environment would release so much energy the result would be the explosion of an atomic bomb (same principle)
- How does one start the fission process?
- Usually a heaver atom is bombarded with neutrons, which is captured by the nucleus of the heaver atom, resulting in a spontaneous chain reaction of intense energy which results in the breakdown of the larger atom to smaller atoms
- When an atomic bomb is detonated over 200 different radioactive by-products are produced
- Energy is released in the form of α ,β , γ , and χ -rays
- Here is an example of how this uncontrolled fission process works using ping pon balls and mousetraps
- In a controlled environment a nuclear reactor undergoes the same process by bombarding Ur or Pu with a neutron. The added neutron increases the increases the atomic number, causing instability of the atom. This causes a splitting into smaller atoms and the release of more energy which results in a chain reaction (the production of these smaller elements are sometimes referred to as by-product materials [NRC])
- Given the following example 235Ur is bombarded with a neutron and then the instability of the atom causes a chain reaction. Note how it eventually yields 99Mo and 131I
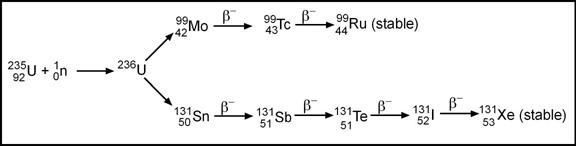
- One of nuclear medicine's major radionuclides is 99Mo which is referred to as "fission Molly" meaning that 99Mo was produced by fission (it can also be procedure with a cyclotron)
- When 235Ur undergoes neutron bombardment 236Ur is created. This causes the chain reaction, resulting in the release of 6 additional neutrons. These 6 neutrons interact with 235Ur creating more 236Ur, then releasing 62 neutrons that bombards more 235Ur, etc. This process continues to repeats itself resulting in a chain reaction that:
- Creates by-product materials
- Produces enough energy such that if not controlled, the proportion of energy would be in the nature of an atomic bomb
- Hence the reaction must be controlled
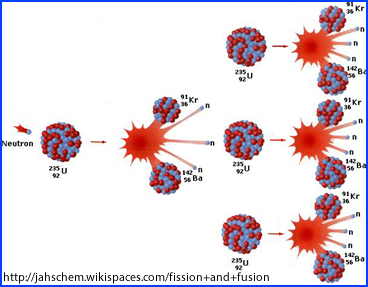
- Here is another example the fission process
- How is this product used in nuclear medicine?
- Via this process of fission there are over 40 different ways in which Ur and Pu can be bombarded to create over 80 different by-products, some which are used in the medical field
- Another important note is that most medical reactors around the world and have a limited life. This is a cause for concern. The inability to have a working medical reactor would cripple to the ability to attain 99Mo for nuclear medicine
- Nuclear reactor - the controlled environment
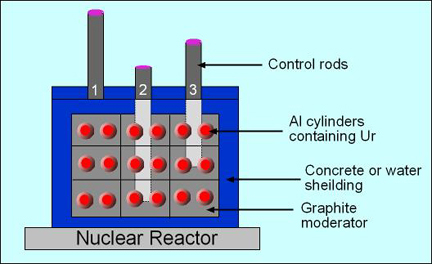
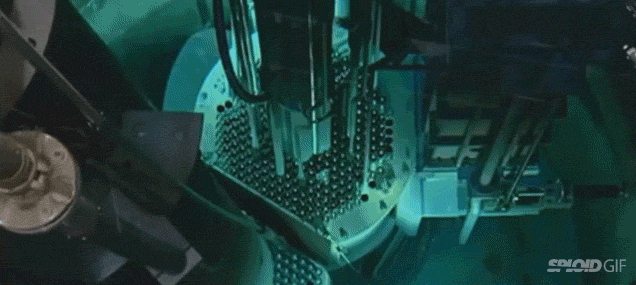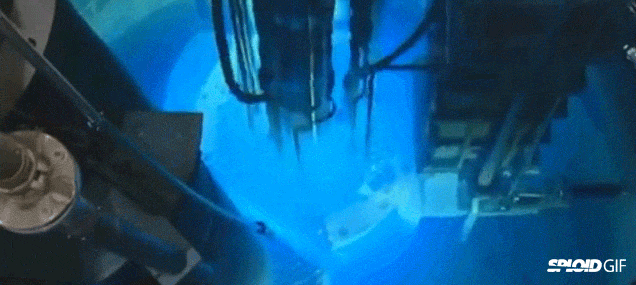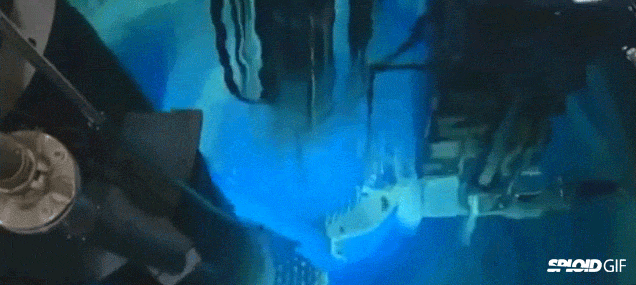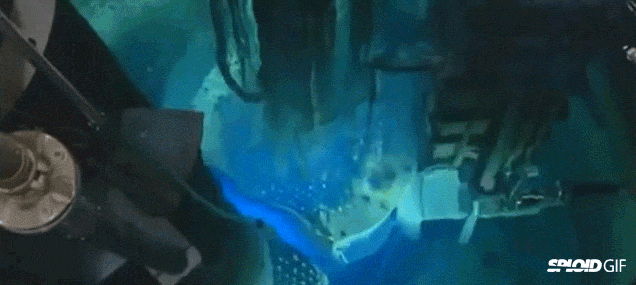
- Image on the left is a diagram of a fission reactor and the image on the right shows what happens when you turn it by initially bombarding it with neutrons. Note the "blue" light
- Parts of a nuclear reactor
- The reactor is either surrounded by water and/or graphite reflector and/or concrete - these products assist in shielding the radiation being produced
- The Al cylinders contain 235Ur, the fuel element
- The modifier is of low Z material that reduces or absorbs the energy of the neutrons being created in the fission process. By reducing the number of neutrons a more controlled and effective fission process can occur
- The control rods are composed of Cd, In, B, or Ag that moves within the Ur core to either slow down or increase the fission reaction. The role is to absorb the production of neutrons therefore slowing down the fission reaction
- Referring to the reactor diagram:
- Rod 2 slows the reaction down the greatest
- Rod 1 is not even in the reactor, hence the greatest amount of fission reaction is occurring that could potentially cause a melt down of the Ur core
- Rod 3 causes more fission to occur when compared to rod 3, but less than rod 1
- Water is also used to cool down the reactor since it generates a lot heat. Failure for water to circulate would cause the reactor to meltdown
- For an actual diagram of what a reactor looks like refer to: https://www.world-nuclear.org/nuclear-essentials/how-does-a-nuclear-reactor-work.aspx
- The NRC also has a site that explains the process of a nuclear reactor: http://www.nrc.gov/reactors.html
- Utilization of neutrons in the production of radioactive materials
- In general, neutrons are an excellent medium to produce radioactive materials
- They have a neutral charge and with little difficulty can penetrate the nucleus of an atom
- Neutron bombardment of a stable element (with smaller AMUs) will add this added neutron to nucleus causing instability of the element
- Neutron activation (neutron capture) - is the example used here
- Example of neutron activation - the formula below where stable 31P is bombarded with a neutron producing 32P. This reaction alas results in the emission of a γ -ray

- This formula can also be written in shorthand method
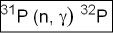
- 31P is referred to as the target
- Inside the parenthesis a neutron, on the left is added to the target
- On the right of the parenthesis a gamma ray is emitted during the process
- 32P is the element that is made after this reaction. Note that the atomic number has increases by 1
- 32P is an isotope of 31P and is radioactive
- What is neutron capture?
- This process involves slow moving (energy) neutrons sometimes referred to as thermal neutrons
- They bombard a target of a stable isotope as noted
- The neutron adds to the atomic number making the element radioactive
- 32P which is a beta pure emitter and is used in certain therapeutic applications
- Usually this process occurs inside a nuclear reactor where stable 31P is placed and then via neutron are captured converting 31P to 32P
- When this process occurs not all 31P converts to 32P, there is always a mixture of both
- The remaining 31P is defined as the carrier of 32P
- 31P cannot be chemically separated from 32P. Why?
- The definition of career and carrier free should be considered when assessing the type(s) of radionuclides present
- Another example with the use of neutron bombardment is the application of using fast neutrons to convert or transmute sulfur to phosphorus (Transmutation)
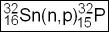
- Unlike thermal neutrons, in this process, we are dealing with faster moving neutron
- Transmutation allows for chemical separation of both elements
- Since we can chemically separate S from P, the process would result in carrier-free 32P
- Any time the term carrier-free is used with a radionuclide of interest it means that there are no other radioisotopes of the same element
Neutron energy range names1
Example of the different types of neutrons used in neutron captivation, and others
| Neutron energy |
Energy range |
| 0.0–0.025 eV |
Cold neutrons |
| 0.025 eV |
Thermal neutrons |
| 0.025–0.4 eV |
Epithermal neutrons |
| 0.4–0.6 eV |
Cadmium neutrons |
| 0.6–1 eV |
EpiCadmium neutrons |
| 1–10 eV |
Slow neutrons |
| 10–300 eV |
Resonance neutrons |
| 300 eV–1 MeV |
Intermediate neutrons |
| 1–20 MeV |
Fast neutrons |
| > 20 MeV |
Ultrafast neutrons |
- Energy ranges neutrons that penetrate the nucleus of an atom
Specific weight
- Specific wight or unit weight is the weight of an element of compound as it relates to its weight per unit volume (ex. mg/mL)
- Why do we need to know the specific weight of radioisotope?
- On application with certain radioactive materials relates to weather or not a metals, poisonous to the human system, such as 201Tl. Knowing the specific weight the level of toxicity can be established
- To much 201Tl in a cardiac exam might produced a toxic effect (not a good idea)
- The good news is that we never even get close to such a toxic level
- Another issue with the administration of radionuclides relates to how some some radioactive materials can complete with a stable element in the human system
- An example of this is 127I (stable) is uses to produce specific hormones within the thyroid
- If we gave too much 123I (radioactive) then we may potentially effect the physiological balance of iodine in a human system
- Let's do some calculations to see if the administration of radioiodine adversely effects iodine physiology
- Does 300 μCi of 123I interfere with physiological process in the human system?
- In order to determine this we must first identify how many atoms are in 300 μ Ci
- Use the following formulas to identify the number atoms in 300 μCi of 123I

- First solve for A by converting activity to dpm

- Calculate λ of 123I

- Now you are ready to solve the equation of N (number of atoms)
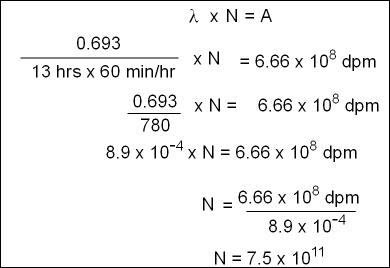
- Once N is known specific weight of 123I can be a calculated with the following
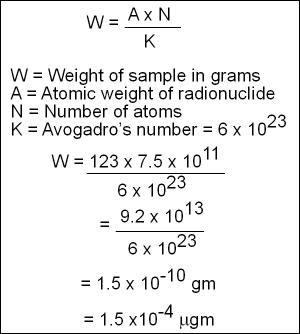
- Hence we know that 300 μCi of 123I will introduce 1.52 x 10-4μg into a human system. Currently a normal daily intake is greater than 382 μg/day. Therefore the amount of radioactive iodine administered will have little to no effect on the physiological process
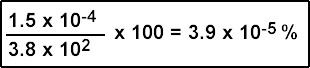
Specific activity
- Specific activity is usually expressed in either mCi/mg or Ci/gm. 123I can first be expressed as follows, in its μCi format
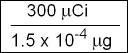
- 300 μCi of 123I is in the μg value and must be converted to mCi/mg format
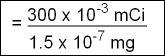
- Hence the specific activity of 300μ Ci of 123I is
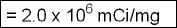
- The specific activity will be found on the radioactive label. Do not mistake this for mCi/mL, which is activity per volume
Return to the beginning of the document
Return to the Table of Contents
If you were to administer 1000 mCi of 67Ga or 111In did you give a toxic levels that reaches an 80% mortality? Go to this link to this link: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0716-97602014000100013. The package inserts for this agents can be found at this link by selecting the isotope: 111InDTPA (package insert) or 67Ga-citrate (package insert). Assume 1 mCi = 1 mg.
Carron, N.J. (2007). An Introduction to the Passage of Energetic Particles Through Matter. p. 308. ]

9/23