- Technetium is a transition metal with atomic number 43 and it belonging to Group VIIB (Mn, Tc, Re) on the periodic table
- Its electronic configuration is 1s22s22p63s23p63d104s24p64d65s1
- Technetium can exist in nine different oxidation states, -1 to +7, that result from the loss of 4d and 5s orbital electrons or the gain of an electron to the 5s orbit
- Stability of these oxidation states depends on
- Chemical environment (pH)
- The type of ligand to which the Tc is chelated to
- The most stable forms are + 7 and +4 states
- Technetium complexes in lower oxidation states (+1, +2, and +3) are stabilized by chelation with various ligands
Tc-99m-sulfur colloid +7 Tc-99m-glucoheptonate (GH) +5 Tc-99m DTPA +4 Tc-99m-DMSA +3 Tc-99m HIDA +3 Tc-99m PYP +3 Tc-99m MDP +3 - Many +5 complexes are found to be stabilized in aqueous media by oxo groups such as TcO3+, trans-TcO2+, and Tc2O34+ that form the central core of the complex
- General comment about oxidation and reduction - is a process in which an element or compound either gain or loss electron(s) during a chemical reaction
- Reduction occurs when an element/compound losses electrons. There are two components: the reduced element and the reducing agent. When the above compound, tin, accepts carbon electrons in the reaction tin dissociates with oxygen forming carbon monoxide. Tin is reduced
- Oxidation occurs when an element gains electrons in which the extra electrons, coming from the oxygen are given to the magnesium.
- Technetium in pertechnetate ion, 99mTcO4-
- Obtained by the elution of the Molly generator
- Its most stable state is Tc+7 where it will only bind to Tc-99m sulfur colloid
- To react with chelation, the Tc+7 state (which is its oxidation after elution)
- Must be reduced to lower oxidation states by reducing agents
- Reducing agents that have been used include stannous salts, concentrated HCl, sodium borohydride, sodium diothionite, ferrous sulfate, ferric chloride plus ascorbic acid, hypophosphorus acid, and hydrazine
- Stannous salts include tartrate, citrate, chloride, fluoride and pyrophosphate, of which stannous chloride (SnCl2.2H2O) is the most common reducing agents
- The reduction reaction that takes place with stannous chloride in acidic medium give
- From the above identify - reduced/reducing agent, chelated production, and HR
- Other oxidation states, individually or in mixture, may be formed under different physicochemical conditions. Example - At neutral pH, stannous ions form colloids which are undesirable result in the chelation process of 99mTc
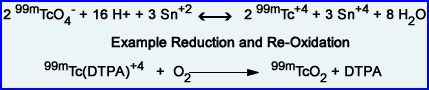
- Complex Formation with Reduced 99mTc
- The reduced species of 99mTc are chemically reactive and form complexes with various chelating agents:
- Reduced 99mTc + Chelating Agent -----> 99mTc-Chelate
- The chelating agent usually donates an ion pair of electrons to form coordinate covalent bonds with 99mTc
- Chelating molecules such as DTPA, macroaggregated albumin (MAA), gluceptate (GH), proteins, and enzymes contain electron donor groups such as - COO-, -OH-, -NH2 and -SH
- Remember the chelating agent - donates
- Pertechnetate in 99mTc Radiopharmaceuticals
- Since not all pertechnetate ions are completely reduced, a small amount of 99mTcO4- is typically present in most 99mTc radiopharmaceuticals
- These free pertechnetate ions are considered radiochemical impurities and may cause artifacts in scintigraphic images. Such as?
- The presence of oxygen, free radicals, or other oxidizing agents in the reagent kit may oxidize the stannous ion to stannic ions which compromises the reduction of Tc7+ resulting in an increase the proportion of free 99mTcO4-
- This can be overcome by
- Adding a sufficient quantity of Sn2+ ions to the kit
- Avoiding oxygen, air, or oxidizing agents to the kit
- Having nitrogen gas atmosphere in the vial/kit. This removes any oxygen from the vial and eliminates an oxidizing agent
- Some kits (MDP, HDP) contain antioxidants (ascorbic acid, gentisic acid) to help further prevent oxidation, especially after the kit has been compounded
- Hydrolysis of Reduced 99mTc and Tin
- In the absence of sufficient chelating agent, reduced technetium can undergo hydrolysis in aqueous solution at pH 6 or higher forming, such as
- These products, if present, can reduce label efficiency and interfere with diagnostic interpretation
- Sn+2 ions can undergo hydrolysis in aqueous solution at pH 6-7 to form insoluble colloids. This can be prevented by adding addition chelating agent

- Preparation of 99mTc Radiopharmaceuticals by Ligand Exchange
- In ligand exchange, transchelation, a 99mTc complex is first formed with a weak chelate in aqueous media. After chelation has occurred a second reaction is started with a stronger chelating agent. Usually heat has to be added (75o C to 100o C) in order for the second chelation event

- EDTA, tartrate, gluconate, and pyrophosphate are all weak chelating agents, whereas ECD (bicisate), isonitile (MIBI), and MAG3 are stronger chelating agents
- Kits containing both weak and strong chelating agents along with stannous ions include:
- Tartrate and MAG3 for renal imaging
- EDTA and ECD for brain imaging
- Citrate and MIBI for myocardial imaging
- The stronger chelating agents are less soluble in aqueous solution and require heating or a longer time to dissolve
- Weaker chelating agents are highly soluble in aqueous solution
- The weaker chelating agent is necessary to stabilize the reduced 99mTc particularly at lower oxidation states
- The absence of the weaker chelating agent would lead to the precipitation of the reduced 99mTc as colloid
- In ligand exchange, transchelation, a 99mTc complex is first formed with a weak chelate in aqueous media. After chelation has occurred a second reaction is started with a stronger chelating agent. Usually heat has to be added (75o C to 100o C) in order for the second chelation event
- Chemistry of 99mTc in Dilute Solutions
- In preparations in which the Sn+2 ion concentration is limited, the total concentration of 99mTc and 99Tc, there may be too high a concentration of 99Tc to the complete reaction
- In such cases (HMPAO) freshly eluted 99mTc is required in order to minimize the amount of 99Tc present
- 99mTc-labeled Peptides and Proteins
- Direct Labeling
- 99mTc can be bound to a colloid that is coated with antibody
- Colloids are taken up by albumin
- 99mTc can be bound to the sulfhydryl groups in the antibody
- A pretinning process whereby the sulfhydryl groups are freed by the reduction of disulfide bonds of the antibody using stannous ion
- Indirect Labeling with 99mTc Complex using Bifunctional Chelators
- 99mTc-chelates are preformed using bifunctional chelating agents [diamidodithio, boronic acid adduct of technetium dioximes (BATO) and cyclam derivatives]
- Used to label proteins by forming bonds between chelating agent and the protein
- Indirect Labeling using Bifunctional Chelators
- A bifunctional chelator (BFC) is conjugated with a macromolecule (protein or peptide) on one side and a metal ion (Tc) by chelation on the other side
- BFCs in use include metallothionein, dithiosemicarbazone and diamide dimercaptide (N2S2)
- The conjugation of the macromolecule takes place between the -NH2 or -SH group of these macromolecules and one of the reactive sites of the BFC
- Direct Labeling
- 99mTc-labeld Red Blood Cells
- In vitro method
- Blood is drawn from the patient, RBCs are primed with Sn+2 and then 99mTcO4- is added
- Sn+2 ion enters the RBC and remains bound to hemoglobin
- Subsequently Tc+7 ion enters the RBC and is reduced by Sn+2
- Reduced-99mTc binds 80% to the beta chain of globin and 20% to heme. Labeling efficiency is >97%
- A commercial kit (UltraTag RBC) is available for this method
- In vivo method
- Sn+2 ions in the form of stannous pyrophosphate are administered IV to the patient
- After a delay of 30 minutes, 99mTcO4- is administered whereby the labeling occurs in the same manner as in the in vivo method
- 10-20 μg/kg body weight of Sn2+ ion is required for optimum labeling
- Labeling efficiency is 80-90%.
- Modified in vitro method
- Sn-PYP is administered IV to the patient in whom an infusion set fitted with a three-way stopcock has been placed
- One port of the stopcock is connected to a syringe containing heparinized saline (10U/mL) and the other port to a syringe containing 99mTcO4-
- Twenty minutes after injection of Sn-PYP, blood is drawn into the 99mTc-syringe and incubated with several gentle mixing over 10 minutes
- Labeled RBCs are injected back into the patient. Label efficiency is >95%
- In vitro method
- 99mTc labeled Leukocytes and Platelets
- 99mTc-HMPAO (Ceretec)
- A neutral lipophilic compound that easily enters the leukocyte by passive diffusion. Freshly prepared 99mTc-HMPAO is added to separated WBCs in plasma/ACD mixture
- After a 15 minute incubation, cells are separated by centrifugation, washed and suspended in plasma for injection. Labeling efficiency is 50-60%
- 99mTc-HMPAO (Ceretec)
- 99mTc Sulesomab (LeukoScan)
- A 99mTc labeled murine antibody fragment (IMMU-MN3) for the nuclear imaging of activated granulocytes
- 99mTc Apcitide (AcuTect)
- A labeled peptide that binds to GPIIb/IIIa adhesion molecule receptors (of the integrin family) found on activated platelets


Return to the beginning of the document
Return to the Table of Content