Types of Radiation – Particles and Rays
- What are the types of emissions from a radionuclide?
- Alpha particle: "
- Beta particle (positron and negatron): $ + and $ -
- Gamma ray/x-ray: ( and P
- Review of the particles
- "
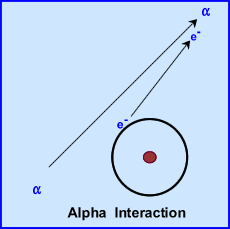
- Alpha particles are ejected from the nucleus of an atom
- Very damaging to life
- When alpha particles are ejected from the nucleus, two neutrons and two protons are released = "
- Kinetic energy released from ejected alpha particle will ionize its surrounding by attracting electrons (see above diagram)V
- Very disruptive and can only travel a short distance in air (4 cm)
- Inhaled, swallowed, or very direct contact can cause significant damage to the surface of an organ/system
- Alpha becomes Helium at rest mass by attracting two electrons
- Rest mass = mass no longer has kinetic energy
- Shielding = absorbing material used to attenuate the ionizing radiation
- Shielding for alpha = as little as a piece of paper
- $ -
- Neutron rich nuclei
- Undergoes a radioactive decay process called beta negative decay
- A neutron rich atom becomes become more stable by the conversion of one of a neutron to a proton
- N ---->P + $- + anti-v
- Anti-neutrino carries away part of the energy of the reaction
- Energy released by this process is shared as kinetic energy between the beta particle and the anti-neutrino
- Beta particle contains numerous energy levels and the energy is shared with anti-neutrino
- As in the case of the above graph where beta and anti-neutrino energies are noted
- In this example 32P has a maximum beta energy of 1.7 MeV (E$-max)
- The average energy particle for beta is know as 1/3Emax
- Whatever energy is not given to the beta particle is given to the anti-neutrino
- Example of a energy distribution for a beta particle of 14C shows is seen below. Note that the beta particle and the anti-neutrino
share is energy based on the amount of energy given
to the beta particle 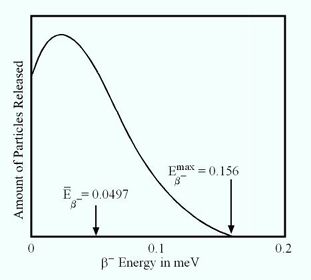
- Average beta-minus given off is 49.7 keV with the maximum beta particle being 156 keV
- What is the energy given to the anti-neutrino when the average beta energy particle is released?
- Average beta-minus particle is the same as 1/3 beta max
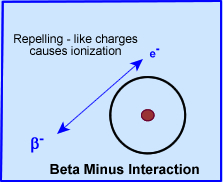
- The $- losses energy as it approaches an outer shell electron, it will repel the outer shell electron, ejecting the electron from its orbit, and causing an ionization event (negative to negative) [see above diagram]
- It should also be noted that E$+max occurs with particles with the same type of energy distribution, except that the particle is positively charged
- At rest mass the $- particle becomes an electron
$- particles can be shielded with either wood and plastic
- Lead may also be used to shield any additional gamma radiation being emitted from the nucleus
- $ +
- proton rich nuclei
- Proton rich atoms decay: P ----> N + $+ + v
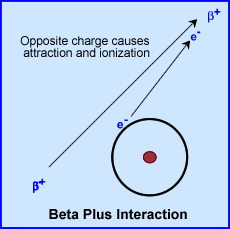
- Positron emission occurs when a proton is converted to a neutron, Beta + (positron), neutrino (v), and gamma ray(s) may also be released
- Once the $+ is ejected by the nucleus the particle attracts electrons (like alpha), thus causing ionization events as it travels (see above diagram)
- Ionization events cause the particle to lose energy until the particle reaches rest mass
- Rest mass is equivalent to a positive electron that causes an annihilation reaction
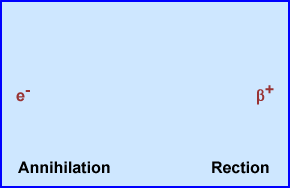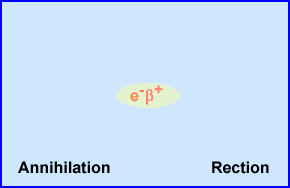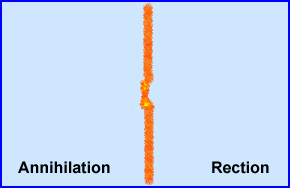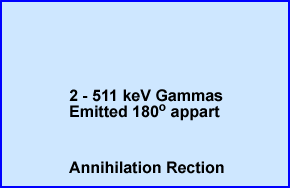
- Annihilation Reaction
- When the $+ reaches rest mass it attracts an e-
- When antimatter, $+, reacts with e- these equal parts of opposite mass annihilated
- Energy is released in the form of two 511 keV ( photons moving in opposite directions)
- Shielding for $+can be achieved with either plastic or wood
- Lead is used to shield gamma rays production
- (
- Occurs when there is additional energy in the nucleus (excited state)
- When the nucleus is in an excited state it has too much energy (see diagram)
- The release of energy as a gamma ray(s) is/are usually the end process that occurs after another decay process
- Example of this process is beta decay
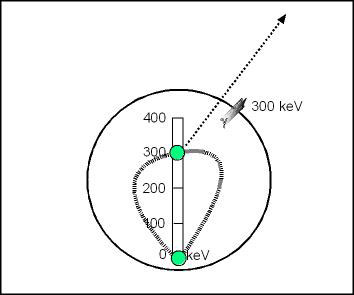
Note the above diagram in which a gamma ray is emitted from the nucleus of an atom
- In a neutron rich atom, a neutron will be converted to a proton and a beta particle will be released
- If there is still too much energy in the nucleus, then the atom will be at a heightened energy state (green ball is raised to 300keV)
- Since the atom is at this heightened energy state, it will release a 300 keV gamma and the atom will return to ground state
An example of this would be I131; following the release of beta particle a 364 keV ( is emitted
- It may also be a process for releasing energy from a nucleus that is metastable nucleus (has too much energy)
- keV - nuclear medicine looks at or images gamma rays
- keV = (kilo electron volt) it the energy given to gamma rays that are released from the nucleus
- An atom may still be radioactive after it releases gamma energy
- P
- Electrons within the obit of an atom receive excess energy, become excited and move to a higher energy state or electron shell
- When the electron returns to its ground state or original electron shell, energy is released in the form x-ray radiation
- What is the difference/similarities between a gamma and an x-ray?
- Gamma rays originate from the nucleus of an atom
- X-rays originate from an electron shell
- Have no weight or charge-like properties
- Are neutral in charge and therefore move farther out than alphas or betas
- Interact with matter - Run into electrons or nuclei in order to lose energy
- Lead density will stop these rays
- At rest mass (loss of keV) nothing is left
Radiation - Interaction With Matter
- Interaction characteristics of particles and rays
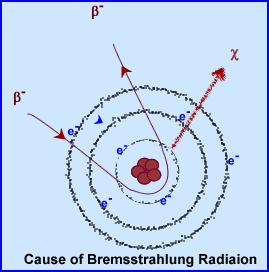
- Bremsstrahlung radiation (in German it means breaking radiation)
- This occurs when accelerated particles encounter material that have a high Z number (density). Example:
- Accelerated particle is a beta
- Example of a high Z number material would be lead
- When a beta particle encounters lead the highly packed molecules cause the beta particle to slow down quickly (interaction between the electrons in the lead and the positively or negativity charged beta particle)
- This causes x-ray emissions
- Should beta decaying radionuclides be stored in lead or plastic?
- Consider a radionuclide that has both beta and gamma decay – what is the best way to shield this type of radioactive material?
- Remember
- - Any time an atom loses an orbiting electron (outer shell or inner shield) it becomes ionized
- Additionally, it will give off radiation, as the outer shell electrons move inward to "cover" the lost electron
- As stated a gamma ray slows down by direct interaction. Let’s take a look at these reactions
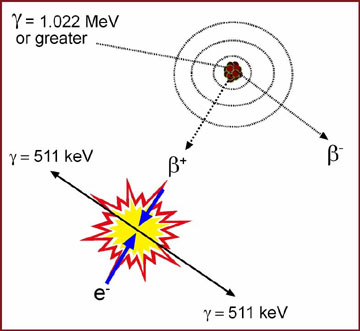
- Gamma rays that are 1.022 MeV or greater in energy (note the diagram below)
- Primary gamma ray interacts near (or in) the nucleus of an atom
- Two particles are produced
- $ + (has applied keV energy)
- $ - (has applied keV energy)
- How due the beta particles lose energy?
- At rest mass the $ - becomes an electron
- At rest mass the $ + interacts with an electron
- Two ( ’s are produced
- In opposite directions (referred to as coincidence)
- Each ( has 511 keV
- Question - Can you account for the total loss of energy in pair production?
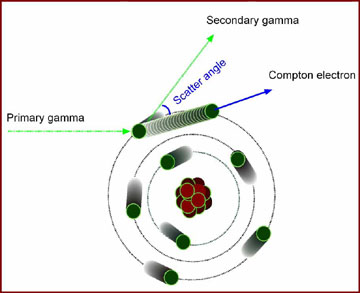
Compton Effect
- A higher energy gamma will interact with the media via a process known as the Compton Effect (see diagram above)
- Primary gamma interacts with an outer shell electron by hitting it
- This creates a secondary gamma (with lower energy) and an ejected electron, known as the Compton electron
- In order to be ejected from its orbit the energy imparted to the outer shell electron must be greater than the binding energy (Note: Energy imparted - binding energy = keV energy of the compton electron)
- The greater degree scatter (max = 180 degrees) the greater the energy imparted to the Compton electron and the less kev energy given to the secondary gamma
- Usually compton interaction occurs several times, until the energy of the gamma ray has been significantly reduced. At this point it will interact photoelectrically
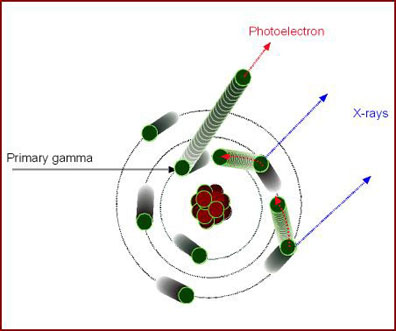
- Photoelectric effect (see diagram below)
- Primary gamma interacts with an inter shell electron
- All the energy is given to the electron creating a photoelectron, eliminating the gamma ray
- keV of the photoelectron is equal to the total energy of the interacting gamma minus the electron's binding energy
- Higher orbiting electrons then move into lower obits creating L or K characteristic radiation (x-ray)
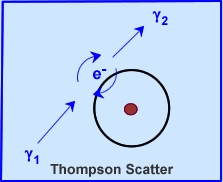
- Thompson or characteristic radiation occurs at very low energy levels, where the binding energy of an electron is greater than the incoming gamma energy
- Gamma ray will interact with the electron
- Electron does not become ejected (because it has a greater energy)
- Secondary gamma ray is emitted with no lost of energy
Other types of gamma/x-ray interaction
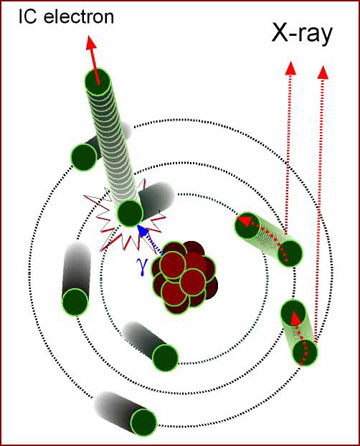
- Internal conversion
- Gamma ray is released from the nucleus
- It interacts with a K-shell electron
- Electron is injected
- Outer shell electrons move into the lower obits to replace the missing electron
- X-rays are produced
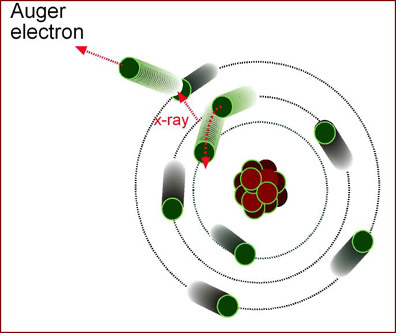
- Auger electron
- Characteristic radiation is emitted from an electron
- X-ray interacts with an outer shell electron
- Electron is removed from obit and it is know as the auger electron
- No secondary x-ray is emitted
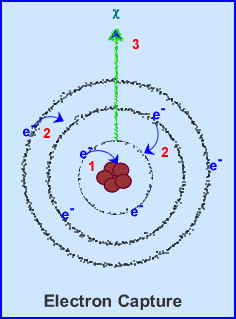
- Electron Capture
(EC)
- Occurs with nuclei that are proton rich, which means that they will decay by positron emission or electron capture
- In EC the following occurs:
- First (1) a K or L-shell electron is captured by the nucleus.
- Second (2) outer shell electrons move in to fill in the vacancy
- Third (3) this causes characteristic radiation to be emitted from the atom
- Proton rich atoms can reach stability either by positron decay or electron capture
- The higher the energy within the nucleus the greater the probability of positron decay. However, the lower the energy of a neutron rich atom, the greater the chance is for electron capture
- 201Tl and 123I are radionuclides in which this occurs
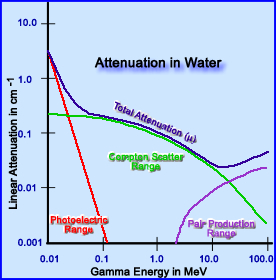
- The above diagram attempts to bring together the process/type of attenuation to the energy of the related gamma
- Photoelectric attenuation occurs between 0 to slightly more than 100 keV
- Compton attenuation occurs anywhere between 0 to 100 MeV, but has its greatest potential at slightly below 100keV and about 20 MeV
- Pair production starts to occurs at 1.024 MeV and continues to increase as the energy gamma increases
- The attenuator being used in this diagram is water
Return to the beginning of the document
Return to the Table of Contents














