- Composition of bone - the bony matrix
- Organic bony matrix contains
- Mucopolysaccharides also known as
- Collagen
- Inorganic bony matrix contains
- Cations: Sr+, K+, Na+, Mg+, and Ca+
- Anions: P-, Cl-, and F-
- Bone is similar to and associated with the connective tissue in the body. It consists of living cells and has a predominant amount of non-living. This is the intercellular substance that has been calcified. between bone and other connective tissue, calcification occurs
- Bone/Osteo components
- Bone composition: organic proteins, collagen, and inorganic minerals
- Approximately 30% organic and 70% inorganic
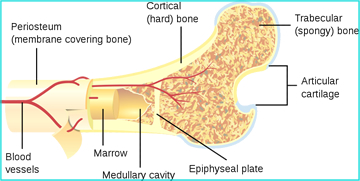
- Organic bone is either cortical (lamellar) bone or trabecular/cancellous (non-lamellar) bone
- Cortical bone, sometimes referred to as "compact bone," is found on the hard surface of bone. This densely ossified material is 80% of the skeletal system weight
- Trabecular (cancellous) bone in is the spongy bone found in the interior portion of the bone. It contains blood forming elements: red and yellow marrow
- Composition
- 25% Water and 30% Organic (cellular elements)
- Of the 30% organic compound 90 - 95% is collagen
- Collagen is mostly protein, in the form of fibers, embedded in a cement type substance. It gives rise to bone strength and flexibility. Furthermore, skin and tendons contain this compound
- 70% of the inorganic material is referred to as your bony matrix (hydroxyapatite). This crystalline structure containing calcium and phosphate ions with univalent anions in a specific ratio. Types of ions include calcium: phosphate, hydroxyl, carbonate, and citrate. To a lesser amounts there is sodium, magnesium, potassium, chloride, and fluorine
- 15% inorganic matter is Calcium
- 12% inorganic matter is Phosphorus--Mainly found as phosphate
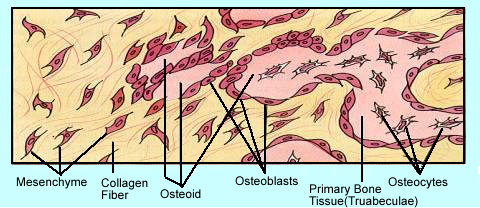
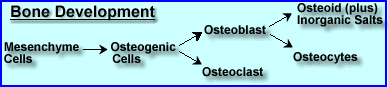
- Bone cells and their development
- Mesenchyme cells are stems cells, the basic unit that generates bone cells and develops into specific types of bone tissue. It can also develop into connective, blood, and lymphatic tissues.
- The other pathways that mesecnchyme stem cell develop are noted
- Mesenchyme cells divide into osteogenic cells that in turn become either osteoblastic or osteoclastic cells
- Osteoblasts are responsible for the formation of the organic portion of the bony matrix. In the process of bone formation osteoblasts secrete organic components "collagen, a ground substance" that become bone tissue
- The secretion is also referred to as osteoids which are composed of fibrous strands (spiral fibers). These strands create a bony network. This network allows for the deposition of calcium and phosphors which further strengthen the substrate. As inorganic salts form on to the fibrous strands, mineralization occurs. These are salts composed of alkaline phosphates. The "web" of organic and inorganic materials becomes the hydroxyapatite crystal (bony matrix) [Ca10(PO4)6(OH)2]. Defined as mineralization.
- Mature osteoblastic cells, over time, can become trapped in the bony matrix and become osteocytes. Osteocytes are considered the "maintaining" component of bone
- Osteocytes control the further development of bone by communicating through channels referred to as dendrites. By secreting sclerostin, osteoblastic activity is reduces and osteoclastic activity increases. What does this do to bone production?
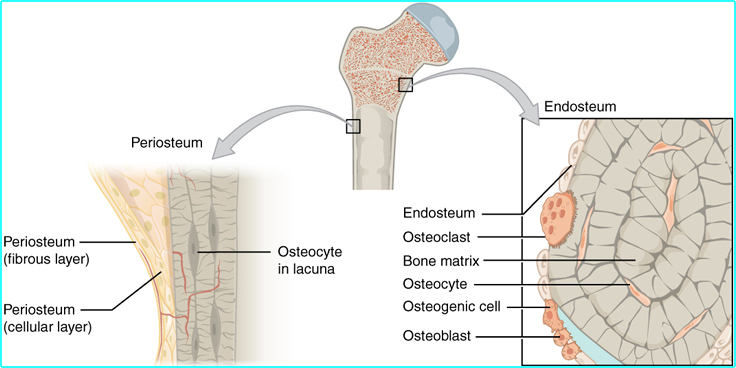
- All the cells mentioned above are noted in the diagram above and the diagram represents a cross section of mature bone
- Finally, osteoclasts arise from osteogenic cells and destroy bone tissue. This processes is referred to as bone remodeling
- Bone development - In this section we will further discuss pathophysiology associated with bone growth and development, osteogenesis or ossification. There are two types of bone growth. Two types of bone formation - endochrondral and intramembranous
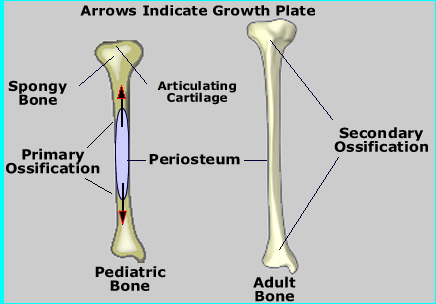
- Endochrondral Ossification - Let's look at long bones development
- The epiphysis contains the epiphyseal plate, where cartilage growth continues to under go mitosis (see below)
- At the growth plate (or part of) is the metaphysis portion, separating the epiphysis from the diaphysis
- This process initially occurs before birth where growth starts at the center of the bone and progresses outward. After birth to about the age of twenty years, two growth plates (epiphysis) emerge and move away from the center
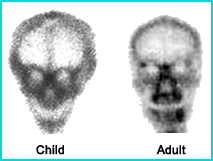
- (Below) On the right is a nine year old child tibia/fibula showing two distinguishable growth plates at either end of the bone
- Long bones continue to lengthen in the process
- The diaphysis is "left behind" and it is here where the osteoblastic cells ossify, cartilage and trabecular bone solidify, forming the bony matrix
- During adolescence the cartilaginous growth and ossification process continues until the early twenties
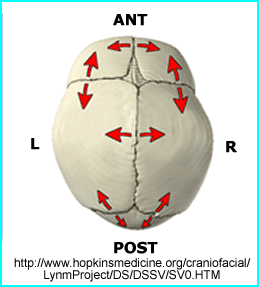
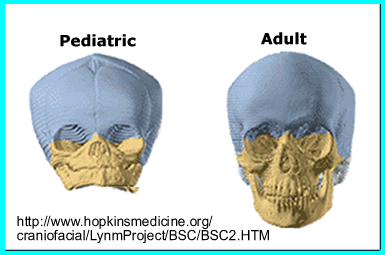
- The skull has two other interesting feature related to bone growth
- One is the manner in which bone growth occurs. Red arrows indicate the angle of bone growth
- Compare the size craniums (adult vs. child)
- Intramembranous ossification - occurs when connective tissue and bony tissue combine to make flat bones, irregular bones, and some of the bones in the skull. This occurs when osteoblastic cells migrate to the connective tissue, then surround themselves with a bony matrix, causing ossification. Once this process is complete the osteoblasts cells become osteocytes.
- Bone remodeling occurs all the time. This process is more prevalent after bone growth has stopped. While this discussion in not limited to the long bone, the long bone is a good example of how bone tissue maintains itself
- Generally, in normal bone, there is balance between osteoblastic/clastic cells. Destroying and replacing bone tissue in a relatively equal manner.
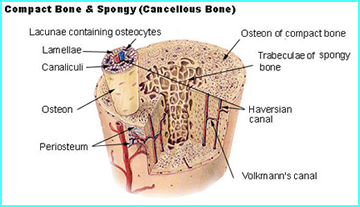
- Osteoblasts work within the periosteum to form bone along its external surface while osteoclasts breakdown bone in the endosteum, inside the bone (see red arrows in diagram)
- This process can
- Increase the bone diameter
- Make the bone thicker
- When everything is in balance bone remodeling causes the bone to remain static
- Above is another look of the different structures that compose bone. Can you associate the roles of the different parts?
- Disease that affects bone density affects bone remodeling, and osteoblast/clast activity
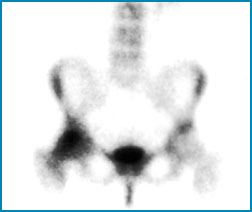
- Paget's occurs when osteoclastic activity destroys too much bone. The osteoblasts then replace the bone it is softer, more cartilaginous tissue. This shows up as increased tracer uptake in a bone scan.
- Osteopenia occurs when there is too much bone destruction and an decrease in bone density. It may sometimes be a precursor to osteoporosis. In a bone scan this will show up has less uptake (radiolucent), unless there is a fx, in which case it will appear hot
- Osteoporosis occurs when the bone losses too much density and becomes fragile. Bone fracture are noted in a bone scan (ex. compression fx in the spine)
- Bone densitometry is used to diagnose the two above mentioned diseases. The procedure defines reduced density in bone. For more information visit https://en.wikipedia.org/wiki/Osteoporosis
- Example of bone density report via DXA or go to website
- Hyperparathyroidism causes excessive bone turnover and may be caused by parathyroid adenoma. Increased levels of Parathyroid hormone (PHA) cause increased calcium turnover in the bone. Example
- The response of bone to any insult, trauma, ischemia, infections, or neoplasm is for it to repair itself by activating osteoblast, osteoclast, and osteocyte
- Radiopharmaceutical development - in the process of developing different bone agents. What do you think would make an idea bone agent? Historically what happened?
- First attempt: 32P and 45Ca
- Pure beta
- Looked for accumulation of activity that indicated increased bone turnover
- No suitable imaging equipment was available (the technology wasn't there) so GM meters were used
- If the technology for a gamma camera was available would, how good would our images be?
- Also think about the radiation absorbed dose (rad) to the patient
- See chart of radionuclides to find energy levels and half-lives
- 47Ca
- Mixed emitter: beta and gamma
- Gamma energy is 1.3 meV
- So what about - radiation dose to the patient, collimation, counting efficiency?
- 85Sr in the early 1960s
- As a Ca analog 85Sr+ is an ionic form exchanges with Ca+ which only takes minutes. This occurs on the surface of the bony matrix and with new bone formation
- It has a 513 keV gamma with a T1/2 of 65 day
- Dose was 100 μCi with a 2 to 7 day time delay before imaging could be started. Why? Answer
- Patient had to be diagnosed with primary cancer before this scan could be ordered
- Issues - radiation exposure and delayed imaging time
- 87mSr
- Is generator produced who's parent is 87Y. It has an 80 hour T1/2
- 388 keV with a T1/2 of 2.9 days
- 1 - 4 mCi dose with a 2 to 3 hour time delay before imaging could be started
- Issues - energy gamma, camera efficiency and collimation, 2 - 3 hour delay is not enough time (too much background)
- Na18F in the late 1960's, early 1970's
- 511 keV with a 1.87 hour T1/2
- Goes to calcium hydroxyapatite and exchanges with hydroxyl group which is similar to F
- At that time availability and transportation as a problem
- Camera technology was another issue - crystal thickness and its high energy photon
- 99mTc phosphate complex: initially developed in 1971
- With the development of the Auger Camera this new radiopharmaceutical had several new properties that greatly improved bone imaging: low energy gamma, increased administering dose, improved count density, and reduced radiation burden
- 99mTcPolyphosphate (PYP)
- This 40 to 55 phosphate chain radiopharmaceutical would break down causing radiocolloid
- Resulted in hepatic uptake
- 99mTc Pyrophosphate (PYR) and 99mTc Ethylenehydroxydiphosphonate (EHDP)
- Shorter phosphate chain which made it more stable
- PYR is naturally occurring and will break down though phosphatase enzymes
- EHDP carbon to carbon bond further improved stability
- Both clear quickly from the blood stream
- Diphosphonates - 99mTcMDP (Methylene diphosphonate) and 99mTcHMDP (Hydroxy methylene diphosphonate)
- The key is to have a faster blood clearance and
- Improved target to background
- The agent of choice maybe MDP (less cost) when compared to HMDP. However, HMDP does clear slightly better and therefore the target to background is improved marginally
- However, from the above comment a comparison of several bone agents, which included MDP and HMDP, it concluded that HMDP "cannot be considered as superior to MDP according to any scintigraphic, clinical or practical criterion.1
- Think - physiology of the radiopharmaceuticals
- Consider vascularity and bone production/turnover
- Uptake is based on radiotracer exchange with ions in the bony matrix, known as heterionic exchange, which is also referred to as chemisorption
- Lytic and blastic lesions should pick up the radiotracer while some types of metastatic disease may not
- When considering a lytic lesion think about bone being destroyed. This occurs when cancer cells spread/invade the bone tissue, resulting in excessive bone turnover. As the bone tries to repair itself the radiotracer is incorporated into the bony matrix
- Blastic lesions is another metastatic process that builds up addition bone onto the bony matrix. As bone is added the radiotracer is incorporated into bone.
- Thought - lytic causes in swiss chess and blastic adds mushrooms
- MDP does a better job in finding blastic lesions, while 18F can identify both lytic and blastic
- Calcium is located on the hydroxyapatite crystal (bony matrix)
- Strontium is an analog of calcium
- 18F exchanges with the OH on the hydroxyapatite crystal
- Phosphate compounds exchange with Ca on the hydroxyapatite crystal
- Osteoclasts and osteoblasts work on the bony matrix
- Uptake of the radiotracers increases when there is high metabolic activity
- Usually due to bone destruction (bone turnover)
- Normal distribution
- Up to 50 percent of the injected bone agent will be eliminated through the kidneys within 3 hours
- Fifty to 60 percent of the radiotracer localizes in bone
- Concentration of the radiotracer is greater in the central skeleton and less in the peripheral bone (chest vs. hands/feet)
- Consider vascular flow and relate to tracer distribution
- Consider imaging central skeleton vs. peripheral bone
 |
 |
 |
 |
 |
 |
Reference:
1 - Which diphosphonate for routine bone (MDP, HDP, or DPD) by Fruhling J, et al.
Return to the Beginning of the Document
Return to the Table of Content
Continue to the Next Lecture