Synthesis of 18FDG
- USP <823> is the regulatory aspect when compounding of PET radio-tracers
- Issue - testing for endotoxins and pathogens on radiopharmaceuticals that have very short lived agents
- LAL - https://pubmed.ncbi.nlm.nih.gov/8441042/
- Systematic review of bacteria testing for PET radiopharmaceuticals - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6406348/
- How might the testing of bacteria and radiopharmaceutical half-life be an issue?
- All cyclotrons now require a manufacturer license. This includes hospital base units
- Nuclear medicine has been reclassified to include the the words molecular imaging. Why?
- Basic agents used in PET are elements related to human life at the cellular level: C, N, O, F
- Relates to its concept of molecular imaging
- Application of Nucleophilic Substitution (important) to the production of 18FDG
- Is a reaction where there is an electron-rich group exchanging with positively charged group of atoms by replacing that group of atoms.
- Consider OSO2F3 (has many electron available in its shell) leaving and the 18F (has one electron shell empty) replacing as seen below
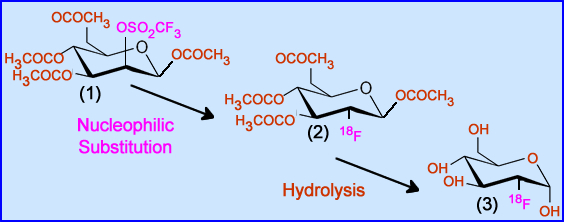
- Mannose Triflate (1) is the initial component needed to develop FDG
- Trimethly sulfate (OSO2CF3) is the leaving group within the carbon ring that is (2) replaced by 18F via nucleophilic substitution
- Acetal groups (H3COCO and OCOCH3) are removed and replaced with OH via hydrolysis, resulting in FDG (3)
- This is accomplished by adding an acid or a base
- Hydrolysis is then applied with heat and HCl
- Note the above reactions are color coded

- Once FDG has be produced
- Acetone and acetanitriles helps clean/remove contaminates from the tracer solution
- Usually occurs in columns
- Micropore filter is used to assist in sterilization and filtering of the final product 18FDG
- The details of compounding via Wikipedia
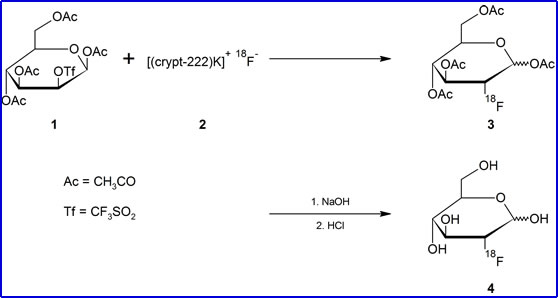
- Separate 18F from solution by trapping it on an ion-exchange column which is then mixed with acetonitrile solution of 2,2,2-cryptand and potassium carbonate
- Evaporation yields [(crypt-222)K+]18F- - This generates #2
- Mannose triflate is then added where 18F reacts with triflate (OSO2CF4) via nucleophilic substitution #3
- HCl causes hydrolysis removing the acetly group #4
- Yields 50-80% FDG
- This is how SNMMI-TS study guide explains the production of FDG (there is some differences)
- 18F is extracted from the targeted solution via a tube and placed into a hot cell for FDG synthesis and place into an ionic exchange cartridge
- 18F is then eluted from the cartridge with potassium carbonate (Kryptofix 222) and acetonitrile and placed into a reactor vessel
- Heating evaporates and removes solvents and the mannose triflate is added
- Heating replaces the triflate group with 18F via nucleophilic substitution
- Results is 18F -FTAg
- Alkaline hydrolysis then removes OCOCH and replaces it with OH producing 18F-FDG
- Sterile water and neutralizing solution is applied which removes the free 18F atoms and any other contaminants
- Finally the solution is passed through a 0.22 μ
- Everything is now done on cassettes that contains all the necessary compounds to label 18F
- Very efficient
- Has all the components needed to tag
- Hook up the lap top to monitor and control the reaction
- Compounding of FDG takes about 30 minutes
- Other types of nucleophilic substitution may have other problems (ex)
- 18Fluorothymidine (FLT) gives a 6 - 12% yield
- Cleanup becomes more difficult: requires pressure device, separations, and then capturing the labeled compound
- Cost $50k to $250k
- QC on FDG (prior to patient use)
- Visual - is it clear and colorless and lacks particles
- Filter member integrity - air pressure test to make sure that the filter isn't broken
- Radiochemical purity - <4% free
- Radionuclide purity - Where are the peaks 511 and 1024 keV are acceptable (why is 1024 keV acceptable?)
- Radionuclide identity - measure decay to make sure that T1/2 is 109 minutes
- pH - must be between 5.5 to 7.5
- Pyrogens/endotoxins - LAL test
- Chemical purity - other impurities are missing when applying gas and thin layer chromatography using Kryptofix 222
- Different products appear at different times on the graph
- Radiolysis
- HPLC - High Pressure Liquid Chromatography
- Sterility - Soy broth to see what might grow (takes 2 weeks) - what's the problem with this testing?
- 2-chloro-2-Deoxy-Dglucose - this can occur when HCl hydrolysis occurs instead of NaOH
- Must be made in a clean room where the compounding room is a class 100 room and the person enters the room from a class 1000 room - sterile environment
Return to the Table of Content
3/24


