Nitrogen is a major limiting nutrient in agriculture (Fixon & West, 2002). The need may be met with chemical fertilizer, but that comes at a steep environmental cost (Vitousek et al, 1997) and is also a major expense that is too high for farmers in many underdeveloped countries (Dixon et al, 2001).
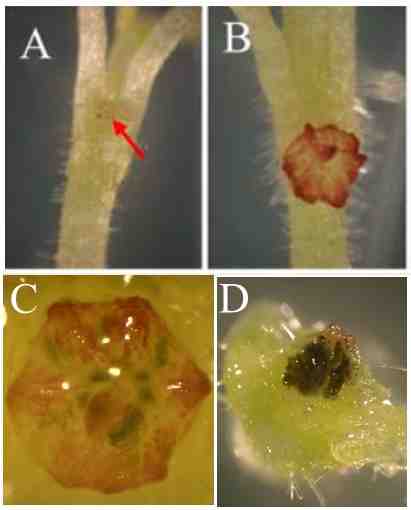
Biological nitrogen fixation addresses both of these concerns. Soil bacteria -- Rhizobia -- in symbiosis with legumes fix atmospheric N2 and provide all the nitrogen needs of the host plants. However these bacteria form symbioses with only narrow group of plants (Wang et al, 2018), not with the cereals (wheat, corn, rice) that make up the bulk of agriculture. In contrast, Nostoc punctiforme, a single strain of N-fixing cyanobacteria, forms symbioses with representatives of a broad range of plants (Santi et al, 2013) (Figure 1).
Why is it that Rhizobia are confined to a thin slice of plant hosts while Nostoc punctiforme is able to interact with representatives from a much more diverse fraction of life? One difference is readily apparent: unlike Rhizobia, Nostoc fixes nitrogen without the need of a special plant-provided environment. It does so by differentiating specialized cells, heterocysts, that protect the nitrogen-fixing apparatus from oxygen. But symbiosis is initiated by the plant, and few choose to do it. What makes these few plants special?

Figure 1. Hosts for cyanobacterial symbionts. (A) Lichen/fungus (from George H Daniel). (B) Azolla/fern (from Onyemeh Nwamarabia). (C) Cycas revoluta/gymnosperm (from Horticultural Impex). (D) Gunnera/angiosperm.