
subsequent printing |
Johann
Heinrich Schulze
c.
1717 - Discovery
that powdered silver nitrate darkened upon exposure to light.
This led to experiments, at first in the flask, later on paper,
wherein stencils were placed in the path of the light rays in
order to produce a negative image upon the silver nitrate.
These
images were temporary, however, because a way had not been found
to stop the action of the light, which always darkened the pictures
to black. His findings were
published in 1719 and reprinted in 1727.
A
scan of the original book is here at the Martin Luther University
|
| I
covered the glass with dark material, exposing
a little part for the free entry of light. Thus
I often wrote names and whole sentences on paper
and carefully cut away the inked parts with a
sharp knife. I struck the paper thus perforated
on the glass with wax. It was not long before
the sun's rays, where they hit the glass through
the cut-out parts of the paper, wrote each word
or sentence on the chalk precipitate so exactly
and distinctly that many who were curious about
the experiment but ignorant of its nature took
occasion to attribute the thing to some sort of
trick." - Johann Heinrich Schulze
(trans. © Robert
Leggat, 2002) |
|
Schulze
at Wikipedia
Silver
and Sunlight at Chemical Heritage Foundation
Photography
Pioneers at FotoArt
|
Invention
of Photo Chemistry at OLinda.com
|
c.
1790 - Thomas Wedgwood, a potter, seeks
to employ photography to transfer designs to pottery
and is the first to attempt to use the camera obscura
to create a photograph on paper. These efforts failed,
but "contact prints" were made of leafs,
etc. by placing the object upon the sensitized paper
- what today we call "photograms".
His collaborator, Humphry Davy, wrote and published
the findings by Wedgwood as, “An Account of
a Method of Copying Paintings upon Glass, and of Making
Profiles, by the Agency of Light upon Nitrate of Silver.
Invented by T. Wedgwood, Esq.” in 1802.
|
|
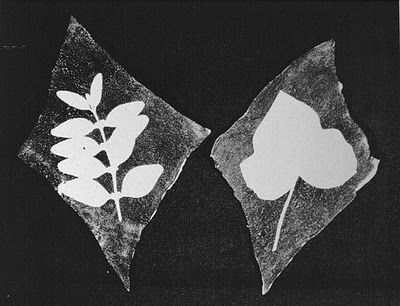 "Shadow
Pictures" or "Sun Pictures" such as
this must be like the
"Shadow
Pictures" or "Sun Pictures" such as
this must be like the
originals by Wedgwood and others, who lacked the ability to
stop
("fix") the continuing action of light on the
silver halide crystals. |
|
|

|
|
In
France, Joseph Niépce, a lithographer,
needed a method to transfer drawings to his printing
plates. He knew that a type of asphalt (bitumen of
Judea) was hardened by the sun. He waxed the paper
of the drawings to make them more translucent &
placed them face down on the plate, which was coated
with the asphaltum. When the light passed through the
paper, it hardened the solution, and where the black
of the drawing ink blocked the light rays, the asphaltum
remained soft & could be removed by a solvent (lavender
oil), leaving behind a negative image. He incised,
by methods of etching, this negative image in order
that it would retain ink for pressure printing. He called
these pictures heliographs ("sun" and "picture").
Circa 1824-26, he placed a plate coated with
his mixture inside a camera obscura and took the first
permanent photograph that did not depend on a contact
method. It was of a barn & some rooftops & took
all day to develop.
|
Joseph
Niépce at University of Texas - Austin
|
In 1829,
Louis J. Daguerre, a painter investigating
photography, approached Niépce in a
quest to share information. Initially suspicious,
after a few years Niépce eventually approached
Daguerre about a business venture, to which he assented.
Niepce died four years later, but Daguerre was able
to release a perfected photographic system by 1839.
This process, called Daguerreotype, involved
exposing a silver coated plate to the fumes of iodine,
which made it vastly more light sensitive than the
asphalt based plates.
Moreover,
a chemical had been discovered (sodium thiosulphate)
to stop the chemical reaction of the plate to incoming
light. The exposed image on the plate was still invisible
(a latent image), but it was "developed"
by exposure to the fumes of mercury, which causes
formation of silver iodide upon the surface of the
plate. The areas that were not struck by light were
washed away by the "fixer". The creation
of an alloy on the surface of the photo gave this
type of picture a bas-relief type surface of great
detail. However, due to metallic glare, it had to
be viewed from the side and copies could not be made.
Obviously,
the fumes involved in the process were very dangerous.
Daguerre
at The Metropolitan Museum
|
|

This process was an improvement in
both detail and speed,
taking about 40 minutes for exposure. |
|
Also
in 1839, only three weeks after Daguerre's announcement
of his method, Henry Fox
Talbot announced in England his "negative-positive"
system. Talbot found that he could slow the effect of
light upon silver nitrate with a salty solution, and
through a friend, had heard about Daguerre's use of
sodium thiosulphate, which halted the process.
Talbot
sensitized paper with silver nitrate, exposed
it to light in a camera obscura, then processed the
image, a "negative reversal" of tone. It was
this picture that became his negative; placed atop a
blank, sensitized sheet of paper and sandwiched between
two planes of glass, the negative sheet created an opposite
image, a "positive", upon the second sheet.
Just as in Niepce's heliotypes, the black "ink",
or, in this case, the darkened silver of the original
photo, blocks light to the lithographic plate or, in
Talbot's method, the second sheet of paper, creating
"white".
Black
and grey tones, obviously, were produced by the "negative",
or original photo, allowing more light to pass through
an unexposed, or less exposed, area.
|
Such
prints were made outdoors by means of sunlight: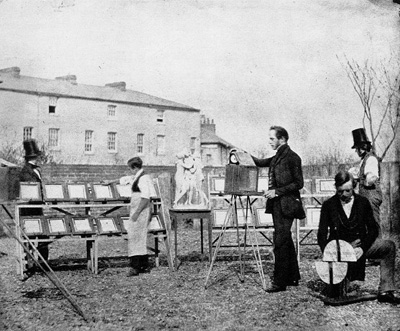
Mass production began with the negative process
|
After
about a year Talbot had also been able to create a latent
image on the negative, meaning there was no need (or
ability, without overexposing the picture!) for "checking"
the progress of the darkening photograph within the
camera or the print between the plates of glass. This
quickened exposure time considerably.
Nevertheless, due to the graininess of the paper, even
when waxed (like Daguerre did) to increase translucency,
the clarity of the pictures was slightly fuzzy, and
a more accurate rendition was sought from photography.
|
Fox
Talbot at De Montfort University
Fox
Talbot at Encyclopedia Britannica
Fox
Talbot Museum
The
Wetplate Collodion Process at :
Alternative
Photography
| Unblinking
Eye
Dry
Plate Photography at The Light Farm
Richard
Leach Maddox:
Microscopy
|
Astrophotography
|
Dry
Plate
|

The Fox Talbott Studio

The first positive print made
from a
photographic negative
|
In
1847, Niepce's nephew introduced the use of a
glass plate as "backing" for the light-sensitive
emulsion, which was ideal for the purpose, being inert,
clear and textureless. The silver nitrate was suspended
in an egg white mixture. In 1846, collodian,
a dangerous mixture containing ether & alcohol,
was developed and exploited by the medical profession
because it dried into a hard, coating-like substance
which could be used to protect exposed wounds.
By
1850, it was used to suspend the silver crystals
in place of the egg white mixture, to superior effect.
However, photos had to be taken while the plate was
still wet (the "wet plate" method)
and processed while still damp. This made traveling
photography a burdensome, dangerous affair. Nevertheless,
exposure times were down to about 5 seconds and the
clarity was excellent.
By
1880, collodion was replaced by animal product
gelatin, still used in modern films to suspend light
sensitive crystals. This allowed the plate to be stored
prior to use, thus creating the "dry plate"
method, freeing photographers from some of their labor.
|
|
Modern
Black and White Film
Silver Bromide
Crystals Before and After Development
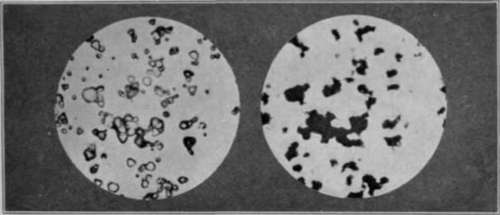
"The Fundamentals of Photography",
by C. E. K. Mees
|
In modern
films, "speed", or light sensitivity,
is determined by the size of the silver bromide crystals.
"Fast" films, therefore, contain large crystals
absorbing more light than smaller crystals ("medium
speed") or very small crystals ("slow film
speed"). The large size of the crystal in the fast
film accelerates exposure time, but possesses a "grainy"
texture. The small crystals in the slow film record
more detail, but lengthen exposure time.
Wikipedia:
Silver
Bromide | Silver
Halide
|
|
In
the bromide crystal, light activates an electron of a
negatively charged bromide ion, which is raised to
a higher energy level in its orbit around the nucleus
and "migrates" toward "impurity elements",
sometimes called "sensitivity specks". The speck
is now negatively charged and attracts a positively charged
free silver ion. The chemical processing of the film exaggerates
this effect, building upon this accumulation of silver.
Areas unexposed by light are washed away in development,
leaving a clear area that will print darkly when light
is passed through it, using, once again, the negative-positive
method first introduced by Talbot. |
|
|
|
| James
Clerk Maxwell, who first understood that light
was part of the electromagnetic spectrum, took the first
color photograph in 1861. He took three separate exposures
of his subject, separately using red, blue and green filters
to admit only light of that wavelength to the photographic
plate. He then took each plate & dyed it the color
of the filter that had produced it. Projecting these three
identical, but differently colored, images onto a screen
and overlapping them, he was able to use the additive
system of color mixing to produce many colors. |
|
|
| Modern
color film uses the same theory, producing three negatives
within a single piece of film by making certain layers
sensitive to only a single color and, similarly, using
filters to block the presence of certain wavelengths at
particular levels. The processing washes away these negatives
& replaces them with colored dyes. Perfectly overlapped,
these three colored levels combine for a single full color
picture. |
|
|
|
lecture
contents
top
|