The
Camera Obscura
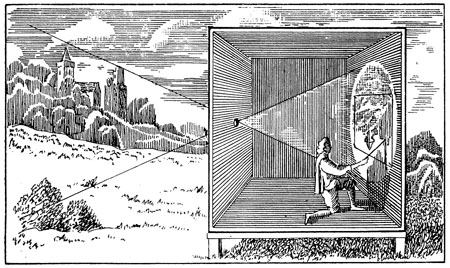
Camera Obscura at the time of the Renaissance
| "Who would believe that so small a space
could contain the image of all the universe? O
mighty process! What talent can avail to penetrate
a nature such as these? What tongue will it be
that can unfold so great a wonder? Verily, none!
This it is that guides the human discourse to
the considering of divine things. Here the figures,
here the colors, here all the images of every
part of the universe are contracted to a point.
O what a point is so marvelous!"
--Leonardo Da Vinci
|
|
Leonardo
- discussion of the camera obscura & his observation of
the spectrum through a prism
(Confiding his discovery only to his notebooks, for fear
that he'd be persecuted as a witch)
Camera
Obscura at National Geographic | History
of Camera Obscura at Obscura Journal
Pinhole
Cameras at Kodak | Pinhole
Cameras at Alternative Photography
Leonardo da
Vinci at: The
Telegraph | Museum
of Science, Boston
|
Agricola's
theory was proven with the development of the first spectroscope
by Dr. Henry Draper in 1872,
made from a cigar box, prism and telescope parts.
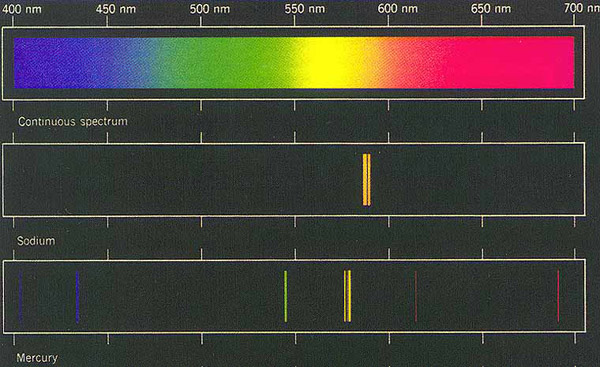
This device reveals the "line spectrum"; for example,
potassium was represented by a single red line, etc.
Line
Spectrum identifications of certain elements & their relationship
to the visible spectrum:
|
Pierre
Janssen and the Solar Eclipse of 1868
The spectrometer (spectroscope) used in solar eclipse
-
Helium is discovered as a single yellow line.
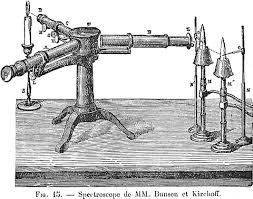
early spectroscope
Janssen
at Wikipedia
|
|
|
| |
|
|
|
|
|
|
Discussion
of the dual nature of light - from an invisible ray
to materialization as a "tiny projectile"
upon contact with matter.
Example
of dual nature -
In experiments, when positrons and electrons of equal size
met, they did not rebound, as expected,
but disappeared in a release of a very short wavelength of
energy that was equal to the energy level of both. This process
has also been reversed.
|
Concept
of "heat" discussed -
molecular movement -
kinetic energy as explanation of heat.
Description of metal in heated, glowing,
melting and boiling stages to illustrate the relationship
between movement and heat/light.
Concept of "stray atom" during boiling stage leads
to brief discussion on particle speed and mass.
|
"Conductors
of heat" - iron & wood contrasted
Description
of gas compression and expansion in refrigerators to help
convey notion of molecular activity producing heat or "less
heat" (cold).
Concept
of "molecular repose" (absolute zero)
Brief
description of superconductivity - property of elements to
better conduct electricity at super cooled temperatures.
|
top
|