| |
Project 1:
Ceramide regulation of the alternative splicing of Bcl-x pre-mRNA
(ongoing for 5 years)
The long-term objectives of this project
focus on the elucidation of the pathways that mediate programmed cell
death (PCD) in response to extracellular agents. Furthermore
and importantly, how dysregulation of apoptotic pathways confers
resistance to PCD and induction of a disease phenotype. In this research
project, our goal is to specifically define the mechanisms involved in
regulating the alternative splicing of the apoptosis regulator, Bcl-x.
Multiple lines of evidence point to a role for the Bcl-2 family in
regulating PCD. Bcl-x(L), a
member of the Bcl-2 family, has been implicated as an inhibitor of PCD,
and many studies have shown that overexpression of Bcl-x(L)
in cells confers PCD resistance to many apoptotic stimuli including
chemotherapy, Fas activation, TNF?, and ?-irradiation. Furthermore, many
cell types spontaneously resistant to chemotherapeutic agents
demonstrate increased levels of Bcl-x(L).
An essential component for understanding
how Bcl-x(L) levels are increased in chemotherapeutic-resistant cancer
cells is to identify and establish how Bcl-x(L) expression is regulated.
To date, the regulation of Bcl-x(L) expression is a complex mechanism
consisting of both transcriptional and post-transcriptional processes.
The post-transcriptional processing of the Bcl-x gene gives rise to at
least 5 different Bcl-x isoforms via alternative splicing (Bcl-x(L),
Bcl-x(s), Bcl-x?, Bcl-x?TM, and Bcl-x?) and studies have shown that
these isoforms have antagonistic functions in some cases. For example,
several studies have clearly demonstrated that the Bcl-x splice variant,
Bcl-x(s), in contrast to Bcl-x(L), promotes apoptosis instead of
inhibiting apoptosis. Bcl-x(s) is produced by activation of an upstream
5’ splice site within the Bcl-x exon 2. Recent studies have shown that
blockage of the downstream Bcl-x(L) specific 5’ splice site in Bcl-x
exon 2 using oligonucleotides induces Bcl-x(s) expression while
downregulating Bcl-x(L) levels and sensitizing A549 lung adenocarcinoma
cells to chemotherapy. Thus, regulation of 5’ splice site selection
within the Bcl-x exon 2 can determine whether a cell is susceptible or
resistant to apoptosis.
Multiple lines of evidence point to roles
for ceramide in regulating apoptosis in response to extracellular
stimuli and published findings from our laboratory have shown that
ceramide regulates the 5’ splice site selection within the Bcl-x exon 2.
We have shown that treatment of A549 lung adenocarcinoma cells with
cell-permeable ceramide and chemotherapies that induce the synthesis of
de novo ceramide downregulated Bcl-x(L)
mRNA and immunoreactive protein levels with a concomitant increase in
mRNA and immunoreactive protein levels of Bcl-x(s).
Downregulation of Bcl-x(L) by
ceramide-induced Bcl-x(s) 5’
splice site activation correlated with increased sensitivity of A549
cells to daunorubicin. Furthermore, A549 cells resistant to
chemotherapeutic agents and cell-permeable ceramides demonstrated
increased Bcl-x(L)
levels due to dysregulated Bcl-x alternative pre-mRNA processing.
In further mechanistic studies by the PI,
it was shown that SR proteins, a family of RNA splicing factors and
substrates for protein phosphatases 1 (a ceramide-activated protein
phosphatases) are dephosphorylated in a time- and dose-dependent manner
by cell- permeable ceramide. Both SR protein dephosphorylation and Bcl-x
alternative splicing were blocked by inhibitors of serine-threonine
protein phosphatases and of the de novo ceramide pathway, suggesting a
role for protein phosphatases 1 (PP1) and endogenous ceramide in
regulating this mechanism. Furthermore, dephosphorylation of SR proteins
has been shown to affect 5’ splice site selection strongly implicating
at least one SR protein family member in regulating Bcl-x 5’ splice site
selection.
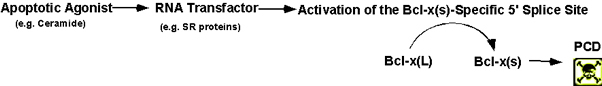
Hypothesis: The above results led
us to hypothesize that RNA transactivating factors, including at least
one SR protein isoform, interacting with specific RNA cis-elements
in Bcl-x pre-mRNA mediate the activation of the Bcl-x exon 2 upstream 5’
splice site (Bcl-x(s)
specific 5’ splice site), thereby, producing Bcl-x(s)
mRNA following ceramide treatment. We are currently testing this
hypothesis.
Highlights of current findings: We
have identified the ceramide-responsive RNA cis-elements
(CRCEs), CRCE 1 and CRCE 2 within Exon 2
of the Bcl-x pre-mRNA. Further studies have identified CRCE 1 as the
critical RNA cis-element for the induction of the Bcl-X(s)
5’ splice site by de novo ceramide. Analysis of RNA trans-factors
that bind to CRCE 1 demonstrated that SAP155, a spliceosomal-associated
protein, specifically bound to CRCE 1 and regulated the activation of
the Bcl-X(s) 5’ splice site by ceramide. Formerly, SAP155 was
thought to only regulate constitutive RNA processing, but these findings
show a clear role for SAP155 in modulating alternative splicing!
Ongoing collaborations with other researchers
(e.g. Dr. Claudio Sette)
have shown other binding partners for SAP155 that are well known
regulators of alternative splicing. Current studies are focusing on
these interactions, the role of the SAP155 phospho-state in this
mechanism, and examining the heterozygous SAP155 knockout mouse for
susceptibility to cancer.
Funding Source: The Veterans
Administration (VA MERIT I)
-Back
to top
Project 2: The
role of ceramide Kinase and ceramide-1-phosphate in eicsosanoid
synthesis. (ongoing for 5 years)
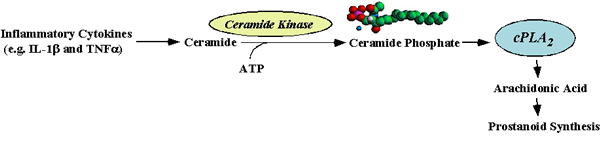
The production of arachidonic acid by
phospholipases is the rate-limiting step in prostaglandin biosynthesis,
and the major phospholipase that regulates prostaglandin synthesis in
response to inflammatory cytokines (e.g.
IL-1beta and TNFalpha)
is type IVA cytosolic phospholipase A2
(cPLA2alpha)
(1). Activation/translocation of
cPLA2alpha in cells requires the association of cPLA2 alpha with
membranes in a Ca2+-dependent manner via a Ca2+-dependent lipid binding
domain (CaLB domain) located near the N-terminus (2,3,4,5). However, the specific membrane lipids that
regulate this binding or whether activation of cPLAalpha 2 also requires
the generation of activating lipids is unknown.
An essential component for understanding
cPLA2alpha activation is to identify and establish the bioactive lipids
responsible for interacting with the CaLB domain and regulating the
membrane association of cPLA2alpha. Ceramide-1-phosphate
(C1P)
is a new addition to bioactive sphingolipids generated by the
phosphorylation of ceramide by ceramide kinase. C1P is one such
potential lipid regulator of cPLA2alpha. Indeed, the main component of
the venom from Loxosceles reclusa (brown recluse spider) is the enzyme sphingomyelinase D
(SMase D) which hydrolyzes sphingomyelin to produce
ceramide-1-phosphate (C1P)
(6). The pathology of a wound generated
from the bite of this spider is that of an intense inflammatory response
mediated by arachidonic acid (AA)
and eicosanoids
(7,8,9). The production of endogenous
C-1-P by the action of SMase D raised the possibility of C-1-P acting as
a patho-physiologic link in the activation of cPLA2alpha and the
inflammatory response mediated by AA and eicosanoids.
Preliminary results from our laboratory
concur with this patho-physiologic link and demonstrate a specific
biology regulated by ceramide-1-phosphate. We found that treatment of
over 12 cell types with C1P
(nanomolar concentrations) induced AA
release and the synthesis of eicosanoids. Further exploration of this
effect demonstrated that C1P induced AA release in various cell types,
and this effect was also lipid-specific as the closely related lipids,
phosphatidic acid, ceramide, diacylglycerol, and sphingosine phosphate
had either minimal or no effects on AA release and prostanoid synthesis.
Preliminary findings also show that C1P induced activation/translocation
of full-length cPLA2alpha as well as the truncated CaLB/C2 domain of
cPLA2alpha. siRNA technology was employed to downregulate cPLA2alpha
which demonstrated that the induction of AA release by C-1-P was
strictly dependent on cPLA2alpha activation. These preliminary findings
also disclose that C-1-P directly binds to cPLA2alpha in a Ca+2 enhanced
manner via the CaLB/C2 domain, and C-1-P also increased the enzymatic
activity of cPLA2alpha in vitro as well as increasing the affinity of
cPLA2 for Ca+2 by approximately 10-fold. Furthermore, studies using
pulse labeling and mass spectrometry demonstrate a marked increase in
C1P concurrent with the release of AA and PGE2 in response to
inflammatory cytokines. siRNA technology to downregulate ceramide kinase
blocked cPLA2alpha activation, AA release and eicosanoid production in
response to inflammatory cytokines, ATP, and A23187 calcium ionophore.
Lastly, our results demonstrate that ceramide-1-phosphate is concurrent
or downstream of calcium mobilization in the activation of cPLA2alpha.
Based on these data, our central
hypothesis is that ceramide phosphate (C-1-P)
produced from the phosphorylation of ceramide by ceramide kinase is an
important mediator of eicosanoid synthesis through activation of
cPLA2alpha in response to inflammatory agonists. To validate our
hypothesis, we are currently answering the following basic questions: 1)
How is ceramide-1-phosphate generated in response to inflammatory
agonists? 2) How is ceramide kinase regulated by inflammatory agonists?
3) What is the interaction site for C1P in the C2 domain of cPLA2alpha?
4) Is the interaction of cPLA2alpha and C1P required for eicosanoid
synthesis is response to agonists? 5) Are there any other enzymes
regulated by C1P in the same manner as cPLA2alpha?
Highlights of current findings:
This project has been steadily advancing over the past few years.
Recently, we have determined the ceramide kinase utilizes ceramide
provided by ceramide transport protein (CERT).
We have localized the enzyme to the trans-Golgi Network
(TGN) as well as early and late endosomes. Furthermore,
activated cPLA2alpha co-localizes with ceramide kinase in cells. Our
laboratory has also undertaken a comprehensive study of
calcium-dependent and –independent mechanisms of CERK activation as they
relate to inflammatory pathways.
Our understanding of the C1P/cPLA2alpha
interaction has also progressed. We have now demonstrated that C1P
enhances the calcium affinity for the enzyme as well as its membrane
affinity. These findings strongly suggest that C1P is a “trigger” for
cPLA2alpha translocation by lowering the dissociation of the enzyme from
PC-rich membranes. Recently, we have also identified several critical
amino acids for C1P interaction in the C2 domain of cPLA2alpha. We are
currently defining whether this interaction site is required for
cPLA2alpha translocation in response to various inflammatory agonists.
Funding Source:
R01 award (1 R01 HL072925-01) from NIH specifically the National
Heart, Lung, and Blood Institute.
-Back
to top
Project
3: The role of the alternative splicing of caspase
9 in oncogenesis.
The long-term objectives of this project
focus on determining how dysregulation of apoptotic pathways confers
resistance to chemotherapy and increases the susceptibility of cells to
oncogenic transformation. Caspase 9 (caspase 9a)
has been shown to be an important factor in the apoptotic pathway and
required for cell death induced by various chemotherapies, stress
agents, and radiation. Studies have shown that the expression of an RNA
splice variant of caspase 9, termed caspase 9b, confers the opposite
effect by inducing resistance to many apoptotic stimuli. The
post-transcriptional processing of caspase 9 pre-mRNA is a complex
mechanism involving the inclusion or exclusion of a four exon cassette
(exons 3, 4, 5, and 6). Inclusion of these four exons
into the mature transcript produces the pro-apoptotic caspase 9 while
exclusion of this cassette produces the anti-apoptotic caspase 9b. The
caspase 9b protein lacks the catalytic domain, but retains all other
amino acid sequence such as the APAF-1 association region. Caspase 9b
competes with the full-length caspase 9 for binding to the apoptosome,
and caspase 9b has also been shown to heterodimerize with full-length
caspase 9, thereby inhibiting the activation of this caspase. Thus,
regulation of the inclusion of this four exon cassette is a critical
factor in determining whether a cell is susceptible or resistant to
apoptosis, and thus oncogenic transformation.
In corroboration with these reports and hypothesis, preliminary
results from the PI’s laboratory demonstrate that the direct modulation
of the alternative splicing of caspase 9 using RNAi and anti-sense RNA
oligonucleotides (ASROs)
significantly affected the susceptibility of A549 cells to daunorubicin
(as measured by WST and clonogenic assays). Induced
expression of caspase 9b by a caspase 9a-specific ASRO in
non-transformed cells also increased the oncogenic ability of c-Myc/H-rasV12
as measured by colony formation in soft agar. In novel mechanistic
studies by the PI, the generation of the lipid second messenger,
ceramide, and the activation of protein phosphatase-1
(PP1) were defined as major components of the signal
transduction pathway that induces the inclusion of the four exon
cassette into the mature caspase 9 transcript. Furthermore, we
demonstrated that SR proteins, a family of RNA splicing factors, were
dephosphorylated in response to the generation of de novo
ceramide in a PP1-dependent manner and within the same time frame as the
inclusion of the four exon cassette into the mature caspase 9
transcript. Preliminary results by the PI’s laboratory also disclose
that the alternative splicing of caspase 9 is intrinsically linked to
the SR protein, SRp30a (ASF/SF2). We found that downregulation of SRp30a using
RNA interference technology (RNAi) dramatically inhibited the inclusion of the 3,
4, 5, 6 exon cassette in the mature caspase 9 transcript. Furthermore,
six possible interaction sites for SRp30a were identified within and
downstream of each exon in the exon 3, 4, 5, and 6 cassette of the
caspase 9 gene. Interestingly, lung adenocarcinoma tumors demonstrated a
dysregulated ratio of caspase 9/caspase 9b that would produce an
anti-apoptotic/chemotherapy resistance phenotype. The culmination of
these data suggest a role for SRp30a and the pre-mRNA processing of
caspase 9 in the apoptotic mechanism of lung adenocarcinoma tumors. In
other mechanistic studies, the protein kinase, CLK/STY, was found to
regulate the phospho-status of SR proteins and the alternative splicing
of caspase 9 in A549 cells. Furthermore, sphingosine-1-phosphate, a
mitogenic bioactive lipid, induced an increase in the phosphorylation of
SR proteins.
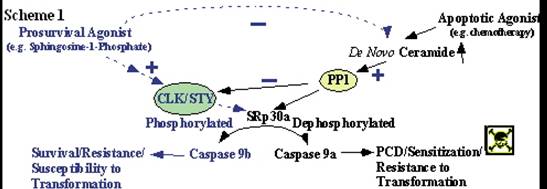
Based on the
above findings, we hypothesize that the alternative splicing of
caspase 9 is a critical factor in determining the susceptibility of
cells to chemotherapy and transformation by oncogenes. Furthermore, we
hypothesize that SRp30a is an important regulator of caspase 9
pre-mRNA processing in response to ceramide via interaction with
specific RNA cis-elements, and that SRp30a regulates the
inclusion of the exon 3, 4, 5, and 6 cassette of caspase 9 via its
phospho-status (Scheme 1).
Lastly, we hypothesize that prosurvival agonists
(e.g. S-1-P) induce the phosphorylation of SRp30a via
activation of CLK/STY, which in turn increases the expression of caspase
9b (Scheme 1).
Highlights of current findings:
We have essentially demonstrated that SRp30a is a required factor for
both basal and ceramide-induced expression of caspase 9a via regulation
of exon inclusion. We have also determined two cis-elements that
regulate ceramide effects on the inclusion of the exon 3,4,5,6 cassette
of caspase 9 pre-mRNA as well as shown that SRp30a interacts
specifically with these RNA cis-elements. We have also determined a
repressor element in exon 3 of the caspase 9 pre-mRNA, but the function
and RNA trans-factors associated with this element are currently
unknown. We have also determined the protein kinase that regulates the
phospho-state of SRp30a. Studies are ongoing to determine whether the
phospho-state of SRp30a has a role in regulating the alternative
splicing of caspase 9. Lastly, we have developed all of the technologies
required to manipulate the alternative splicing of caspase 9 and are
examining the role of this mechanism in oncogenesis and sensitivity of
cells to various chemotherapies.
We believe these studies will demonstrate that the alternative
splicing of caspase 9 is a key mechanism for regulating the
susceptibility of cells to chemotherapy-induced cell death and oncogenic
transformation. These studies will also largely define the signal
transduction pathway leading to the inclusion of the exon 3, 4, 5, and 6
cassette of caspase 9 in response to apoptotic agonists. Furthermore,
these studies will begin to define factors involved in the signal
transduction pathway that regulates the pro-survival activation of the
exclusion the exon 3, 4, 5, and 6 cassette of caspase 9. This
cannot be understated because the definition of these signal
transduction pathways creates, not one, but many new targets, for
anti-cancer therapies. These are exciting studies, and our laboratory
group looks forward to pursuing the identification of both the apoptotic
and pro-survival pathways of signal transduction that regulate the fate
of a cell, and thus, a whole organism.
Funding
Source: R01 award
(1 R01 CA117950-02) from
NIH specifically the National Cancer Institute.
-Back
to top
Thanks to our collaborators without
whose help, these studies could not be done!
1)
Dr. Alfred H. Merrill, Jr. at Georgia Tech
Dr. Cameron Sullards
Samuel Kelly
Elaine Wang
Jeremy Allegood
2)
Dr. Sarah Spiegel at VCU
Dr. Shawn Payne
Dr. Mike Maceyka
3)
Dr. Yusuf Hannun at MUSC
Dr. Ben Pettus
Patrick Roddy
Dr. Alicja Bielawska
Dr. Zsdislaw Szulc
4)
Dr. Besim Ogretmen at MUSC
5)
Dr. Lina Obeid at MUSC
6)
Dr. Wonhwa Cho at Univ. of Illinois at Chicago
Dr. Rob Stahelin
(currently moving into an independent position!)
7)
All current and past Chalfant Lab Members!!! |
|
|



